In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Copper(I) iodide is an inorganic compound with the chemical formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.
Inorganic Crystal Structure Database (ICSD) is a chemical database founded in 1978 by Günter Bergerhoff at the University of Bonn in Germany and I. D. Brown at McMaster University in Canada. It is now produced by FIZ Karlsruhe in Europe and the U.S. National Institute of Standards and Technology. It seeks to contain information on all inorganic crystal structures published since 1913, including pure elements, minerals, metals, and intermetallic compounds. ICSD contains over 210,000 entries as of December 2020 and is updated twice a year.

Cupferron is jargon for the ammonium salt of the conjugate base derived from N-nitroso-N-phenylhydroxylamine. It once was a common reagent for the complexation of metal ions, being of interest in the area of qualitative inorganic analysis. Its formula is NH4[C6H5N(O)NO]. The anion binds to metal cations through the two oxygen atoms, forming five-membered chelate rings.

CrystEngComm is a peer-reviewed online-only scientific journal publishing original research and review articles on all aspects of crystal engineering including properties, polymorphism, target materials, and crystalline nanomaterials. It is published biweekly by the Royal Society of Chemistry and the editor-in-chief is Pierangelo Metrangolo. According to the Journal Citation Reports, the journal has a 2021 impact factor of 3.756. CrystEngComm has a close association with the virtual web community, CrystEngCommunity.
Acta Crystallographica is a series of peer-reviewed scientific journals, with articles centred on crystallography, published by the International Union of Crystallography (IUCr). Originally established in 1948 as a single journal called Acta Crystallographica, there are now six independent Acta Crystallographica titles:

Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials publishes scientific articles on structural science. According to the journal: "Knowledge of the arrangements of atoms, including their temporal variations and dependencies on temperature and pressure, is often the key to understanding physical and chemical phenomena and is crucial for the design of new materials and supramolecular devices." It was formed in 1968 when the journal Acta Crystallographica was split into two parts and has been published for the International Union of Crystallography under the following titles:

Acta Crystallographica Section D: Structural Biology publishes articles covering all areas of structural biology, including biomolecular structures determined by NMR and cryo-EM as well as crystallography, and the methods used to obtain them. The journal was launched in 1993 as Acta Crystallographica Section D: Biological Crystallography with Jenny Glusker as the founding Editor. In 2003, Ted Baker and Zbigniew Dauter took over the editorship of the journal. The current Editors are Elspeth Garman, Randy J. Read and Charles S. Bond. In 2016, the title was changed to Acta Crystallographica Section D: Structural Biology to reflect the expanded scope of the journal.

Acta Crystallographica Section C: Structural Chemistry is a journal for the rapid publication of research with structural content relating to the chemical sciences.
A crystallographic database is a database specifically designed to store information about the structure of molecules and crystals. Crystals are solids having, in all three dimensions of space, a regularly repeating arrangement of atoms, ions, or molecules. They are characterized by symmetry, morphology, and directionally dependent physical properties. A crystal structure describes the arrangement of atoms, ions, or molecules in a crystal..

Acta Crystallographica Section A: Foundations and Advances is a peer-reviewed structural science journal published bimonthly by the International Union of Crystallography. It contains papers describing fundamental developments in structural science. It was founded in 1967 when Acta Crystallographica was split into two sections, and was initially titled Acta Crystallographica Section A: Crystal Physics, Diffraction, Theoretical and General Crystallography. The journal's name changed in 1982 to Acta Crystallographica Section A: Foundations of Crystallography. The journal adopted its current title in 2013.
Acta Crystallographica Section F is a rapid structural biology communications journal. It publishes short papers on biological structures and any aspects of structural biology.

Monofluorophosphate is an anion with the formula PO3F2−, which is a phosphate group with one oxygen atom substituted with a fluoride atom. The charge of the ion is −2. The ion resembles sulfate in size, shape and charge, and can thus form compounds with the same structure as sulfates. These include Tutton's salts and langbeinites. The most well-known compound of monofluorophosphate is sodium monofluorophosphate, commonly used in toothpaste.

Transition metal pyridine complexes encompass many coordination complexes that contain pyridine as a ligand. Most examples are mixed-ligand complexes. Many variants of pyridine are also known to coordinate to metal ions, such as the methylpyridines, quinolines, and more complex rings.
In Chemistry, a kryptoracemic compound or kryptoracemate is a racemic compound crystallizing in a Sohncke space group.

In chemistry, a transition metal chloride complex is a coordination complex that consists of a transition metal coordinated to one or more chloride ligand. The class of complexes is extensive.
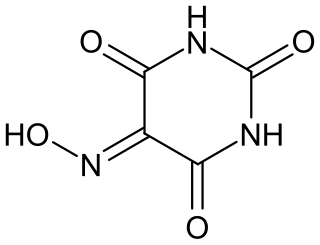
Violuric acid is an organic compound with the formula HON=C(CONH)2CO. It crystallizes as white or off-white monohydrate. The compound has attracted attention because its salts are deeply colored.
Robert Day Shannon is a retired research chemist formerly at DuPont de Nemours, Inc.
Alexander Frank Wells, or A. F. Wells, was a British chemist and crystallographer. He is known for his work on structural inorganic chemistry, which includes the description and classification of structural motifs, such as the polyhedral coordination environments, in crystals obtained from X-ray crystallography. His work is summarized in a classic reference book, Structural inorganic chemistry, first appeared in 1945 and has since gone through five editions. In addition, his work on crystal structures in terms of nets have been important and inspirational for the field of metal-organic frameworks and related materials.
Urea can crystallise with other compounds. These can be called urea adducts or if a solvent is involved, a urea solvate, and the process is called urea extraction crystallization. Urea can also be a neutral ligand if it is coordinated to a central metal atom. Urea can form hydrogen bonds to other oxygen and nitrogen atoms in the substance it crystallises with. This stiffens the solid and raises the melting point. T












