Actinium is a chemical element with the symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. Actinium gave the name to the actinide series, a set of 15 elements between actinium and lawrencium in the periodic table. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.

Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was named after the Americas.
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.
A chemical element is a chemical substance that cannot be broken down into other substances. The basic particle that constitutes a chemical element is the atom, and chemical elements are distinguished from each other by the number of protons in the nuclei of their atoms. This is in contrast to chemical compounds and mixtures.

Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

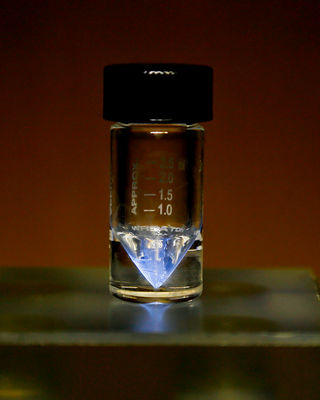
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory, by bombarding curium with alpha particles. It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye. The element was named after the university and the U.S. state of California.

Lawrencium is a synthetic chemical element with the symbol Lr and atomic number 103. It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radioactive metal, lawrencium is the eleventh transuranic element and the last member of the actinide series. Like all elements with atomic number over 100, lawrencium can only be produced in particle accelerators by bombarding lighter elements with charged particles. Fourteen isotopes of lawrencium are currently known; the most stable is 266Lr with half-life 11 hours, but the shorter-lived 260Lr is most commonly used in chemistry because it can be produced on a larger scale.

Mendelevium is a synthetic element with the symbol Md and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in macroscopic quantities by neutron bombardment of lighter elements. It is the third-to-last actinide and the ninth transuranic element. It can only be produced in particle accelerators by bombarding lighter elements with charged particles. Seventeen isotopes are known; the most stable is 258Md with half-life 51 days; however, the shorter-lived 256Md is most commonly used in chemistry because it can be produced on a larger scale.

The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomic numbers. The table is divided into four roughly rectangular areas called blocks. The rows of the table are called periods, and the columns are called groups. Elements from the same group of the periodic table show similar chemical characteristics. Trends run through the periodic table, with nonmetallic character increasing from left to right across a period, and from down to up across a group, and metallic character increasing in the opposite direction. The underlying reason for these trends is electron configurations of atoms. The periodic table exclusively lists electrically neutral atoms that have an equal number of positively charged protons and negatively charged electrons and puts isotopes at the same place. Other atoms, like nuclides and isotopes, are graphically collected in other tables like the tables of nuclides.

Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided.
The transuranium elements are the chemical elements with atomic numbers greater than 92, which is the atomic number of uranium. All of these elements are unstable and decay radioactively into other elements. With the exception of neptunium and plutonium, all do not occur naturally on Earth and are synthetic.
An extended periodic table theorises about chemical elements beyond those currently known in the periodic table and proven. As of 2023, the element with the highest atomic number known is oganesson (Z = 118), which completes the seventh period (row) in the periodic table. All elements in the eighth period and beyond thus remain purely hypothetical.

Dmitri Mendeleev published a periodic table of the chemical elements in 1869 based on properties that appeared with some regularity as he laid out the elements from lightest to heaviest. When Mendeleev proposed his periodic table, he noted gaps in the table and predicted that then-unknown elements existed with properties appropriate to fill those gaps. He named them eka-boron, eka-aluminium, eka-silicon, and eka-manganese, with respective atomic masses of 44, 68, 72, and 100.
A period 7 element is one of the chemical elements in the seventh row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases: a new row is begun when chemical behavior begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The seventh period contains 32 elements, tied for the most with period 6, beginning with francium and ending with oganesson, the heaviest element currently discovered. As a rule, period 7 elements fill their 7s shells first, then their 5f, 6d, and 7p shells in that order, but there are exceptions, such as uranium.

The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inserting blank cells, so that rows (periods) and columns (groups) show elements with recurring properties. For example, all elements in group (column) 18 are noble gases that are largely—though not completely—unreactive.
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the last actinide is lawrencium. By definition, superheavy elements are also transuranium elements, i.e., having atomic numbers greater than that of uranium (92). Depending on the definition of group 3 adopted by authors, lawrencium may also be included to complete the 6d series.

Alternative periodic tables are tabulations of chemical elements differing in their organization from the traditional depiction of the periodic system.

Organoactinide chemistry is the science exploring the properties, structure and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.

Larned (Larry) Brown Asprey was an American chemist noted for his work on actinide, lanthanide, rare-earth, and fluorine chemistry, and for his contributions to nuclear chemistry on the Manhattan Project and later at the Los Alamos National Laboratory.
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-block, p-block, d-block, f-block and g-block.













