
The citric acid cycle—also known as the Krebs cycle, Szent–Györgyi–Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a "cycle", it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.
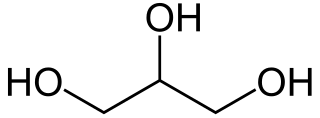
Glycerol, also called glycerine or glycerin, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.
In organic chemistry, a tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 (aldotetroses) or a ketone group in position 2 (ketotetroses).

Novobiocin, also known as albamycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

Glucose-6-phosphate dehydrogenase (G6PD or G6PDH) (EC 1.1.1.49) is a cytosolic enzyme that catalyzes the chemical reaction

Platensimycin, a metabolite of Streptomyces platensis, is an antibiotic, which acts by blocking the enzymes β-ketoacyl-(acyl-carrier-protein ) synthase I/II (FabF/B).
sn-Glycerol 3-phosphate is the organic ion with the formula HOCH2CH(OH)CH2OPO32-. It is one of two stereoisomers of the ester of dibasic phosphoric acid (HOPO32-) and glycerol. It is a component of bacterial and eukaryotic glycerophospholipids. From a historical reason, it is also known as L-glycerol 3-phosphate, D-glycerol 1-phosphate, L-α-glycerophosphoric acid.

Glycerol kinase, encoded by the gene GK, is a phosphotransferase enzyme involved in triglycerides and glycerophospholipids synthesis.

The glycerol-3-phosphate shuttle is a mechanism used in skeletal muscle and the brain that regenerates NAD+ from NADH, a by-product of glycolysis. NADH is a reducing equivalent that stores electrons generated in the cytoplasm during glycolysis. NADH must be transported into the mitochondria to enter the oxidative phosphorylation pathway. However, the inner mitochondrial membrane is impermeable to NADH and only contains a transport system for NAD+. Depending on the type of tissue either the glycerol-3-phosphate shuttle pathway or the malate–aspartate shuttle pathway is used to transport electrons from cytoplasmic NADH into the mitochondria.

6-Phosphogluconolactonase (EC 3.1.1.31, 6PGL, PGLS, systematic name 6-phospho-D-glucono-1,5-lactone lactonohydrolase) is a cytosolic enzyme found in all organisms that catalyzes the hydrolysis of 6-phosphogluconolactone to 6-phosphogluconic acid in the oxidative phase of the pentose phosphate pathway:

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.
The methylglyoxal pathway is an offshoot of glycolysis found in some prokaryotes, which converts glucose into methylglyoxal and then into pyruvate. However unlike glycolysis the methylglyoxal pathway does not produce adenosine triphosphate, ATP. The pathway is named after the substrate methylglyoxal which has three carbons and two carbonyl groups located on the 1st carbon and one on the 2nd carbon. Methylglyoxal is, however, a reactive aldehyde that is very toxic to cells, it can inhibit growth in E. coli at milimolar concentrations. The excessive intake of glucose by a cell is the most important process for the activation of the methylglyoxal pathway.

In enzymology, a glycerol-3-phosphate dehydrogenase (NAD+) (EC 1.1.1.8) is an enzyme that catalyzes the chemical reaction

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies.
Fructolysis refers to the metabolism of fructose from dietary sources. Though the metabolism of glucose through glycolysis uses many of the same enzymes and intermediate structures as those in fructolysis, the two sugars have very different metabolic fates in human metabolism. Under one percent of ingested fructose is directly converted to plasma triglyceride. 29% - 54% of fructose is converted in liver to glucose, and about a quarter of fructose is converted to lactate. 15% - 18% is converted to glycogen. Glucose and lactate are then used normally as energy to fuel cells all over the body.
Glyceroneogenesis is a metabolic pathway which synthesizes glycerol 3-phosphate from precursors other than glucose. Usually, glycerol 3-phosphate is generated from glucose by glycolysis, in the liquid of the cell's cytoplasm. Glyceroneogenesis is used when the concentrations of glucose in the cytosol are low, and typically uses pyruvate as the precursor, but can also use alanine, glutamine, or any substances from the TCA cycle. The main regulator enzyme for this pathway is an enzyme called phosphoenolpyruvate carboxykinase (PEPC-K), which catalyzes the decarboxylation of oxaloacetate to phosphoenolpyruvate. Glyceroneogenesis is observed mainly in adipose tissue, and in the liver. A significant biochemical pathway regulates cytosolic lipid levels. Intense suppression of glyceroneogenesis may lead to metabolic disorders such as type 2 diabetes.
Glycerol-3-phosphate dehydrogenase (EC 1.1.5.3 is an enzyme with systematic name sn-glycerol 3-phosphate:quinone oxidoreductase. This enzyme catalyses the following chemical reaction

Estramustine is an estrogen and cytostatic antineoplastic agent which was never marketed. It is a carbamate derivative of estradiol and acts in part as a prodrug of estradiol in the body. Estramustine phosphate, the C17β phosphate ester of estramustine and a prodrug of estramustine, estromustine, estradiol, and estrone, is marketed and used in the treatment of prostate cancer.

Glycerol-3-phosphate dehydrogenase 1 is an enzyme that is encoded by the GPD1 gene in humans.

C-1027 or lidamycin is an antitumor antibiotic consisting of a complex of an enediyne chromophore and an apoprotein. It shows antibiotic activity against most Gram-positive bacteria. It is one of the most potent cytotoxic molecules known, due to its induction of a higher ratio of DNA double-strand breaks than single-strand breaks.














