Related Research Articles
In physiology, nociception, also nocioception; from Latin nocere 'to harm/hurt') is the sensory nervous system's process of encoding noxious stimuli. It deals with a series of events and processes required for an organism to receive a painful stimulus, convert it to a molecular signal, and recognize and characterize the signal to trigger an appropriate defensive response.
An evoked potential or evoked response is an electrical potential in a specific pattern recorded from a specific part of the nervous system, especially the brain, of a human or other animals following presentation of a stimulus such as a light flash or a pure tone. Different types of potentials result from stimuli of different modalities and types. Evoked potential is distinct from spontaneous potentials as detected by electroencephalography (EEG), electromyography (EMG), or other electrophysiologic recording method. Such potentials are useful for electrodiagnosis and monitoring that include detections of disease and drug-related sensory dysfunction and intraoperative monitoring of sensory pathway integrity.

The brainstem is the stalk-like part of the brain that interconnects the cerebrum and diencephalon with the spinal cord. In the human brain, the brainstem is composed of the midbrain, the pons, and the medulla oblongata. The midbrain is continuous with the thalamus of the diencephalon through the tentorial notch.

In neuroanatomy, the trigeminal nerve (lit. triplet nerve), also known as the fifth cranial nerve, cranial nerve V, or simply CN V, is a cranial nerve responsible for sensation in the face and motor functions such as biting and chewing; it is the most complex of the cranial nerves. Its name (trigeminal, from Latin tri- 'three', and -geminus 'twin') derives from each of the two nerves (one on each side of the pons) having three major branches: the ophthalmic nerve (V1), the maxillary nerve (V2), and the mandibular nerve (V3). The ophthalmic and maxillary nerves are purely sensory, whereas the mandibular nerve supplies motor as well as sensory (or "cutaneous") functions. Adding to the complexity of this nerve is that autonomic nerve fibers as well as special sensory fibers (taste) are contained within it.
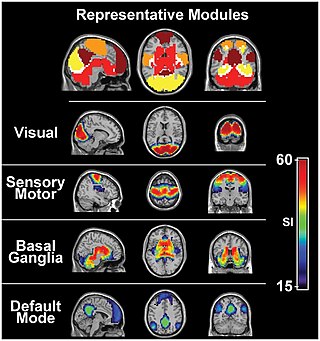
The sensory nervous system is a part of the nervous system responsible for processing sensory information. A sensory system consists of sensory neurons, neural pathways, and parts of the brain involved in sensory perception and interoception. Commonly recognized sensory systems are those for vision, hearing, touch, taste, smell, balance and visceral sensation. Sense organs are transducers that convert data from the outer physical world to the realm of the mind where people interpret the information, creating their perception of the world around them.
A thermoreceptor is a non-specialised sense receptor, or more accurately the receptive portion of a sensory neuron, that codes absolute and relative changes in temperature, primarily within the innocuous range. In the mammalian peripheral nervous system, warmth receptors are thought to be unmyelinated C-fibres, while those responding to cold have both C-fibers and thinly myelinated A delta fibers. The adequate stimulus for a warm receptor is warming, which results in an increase in their action potential discharge rate. Cooling results in a decrease in warm receptor discharge rate. For cold receptors their firing rate increases during cooling and decreases during warming. Some cold receptors also respond with a brief action potential discharge to high temperatures, i.e. typically above 45 °C, and this is known as a paradoxical response to heat. The mechanism responsible for this behavior has not been determined.

A nociceptor is a sensory neuron that responds to damaging or potentially damaging stimuli by sending "possible threat" signals to the spinal cord and the brain. The brain creates the sensation of pain to direct attention to the body part, so the threat can be mitigated; this process is called nociception.

The spinothalamic tract is a part of the anterolateral system or the ventrolateral system, a sensory pathway to the thalamus. From the ventral posterolateral nucleus in the thalamus, sensory information is relayed upward to the somatosensory cortex of the postcentral gyrus.
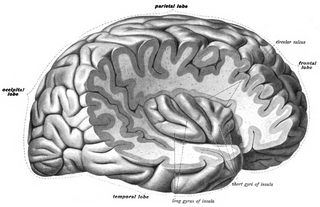
The insular cortex is a portion of the cerebral cortex folded deep within the lateral sulcus within each hemisphere of the mammalian brain.

Brown-Séquard syndrome is caused by damage to one half of the spinal cord, i.e. hemisection of the spinal cord resulting in paralysis and loss of proprioception on the same side as the injury or lesion, and loss of pain and temperature sensation on the opposite side as the lesion. It is named after physiologist Charles-Édouard Brown-Séquard, who first described the condition in 1850.

The anterior white commissure is a bundle of nerve fibers which cross the midline of the spinal cord just anterior to the gray commissure. A delta fibers and C fibers carrying pain sensation in the spinothalamic tract contribute to this commissure, as do fibers of the anterior corticospinal tract, which carry motor signals from the primary motor cortex.

Group C nerve fibers are one of three classes of nerve fiber in the central nervous system (CNS) and peripheral nervous system (PNS). The C group fibers are unmyelinated and have a small diameter and low conduction velocity, whereas Groups A and B are myelinated. Group C fibers include postganglionic fibers in the autonomic nervous system (ANS), and nerve fibers at the dorsal roots. These fibers carry sensory information.
The ventrobasal complex (VB) is a relay nucleus of the thalamus for nociceptive stimuli received from nociceptive nerves. The VB consists of the ventral posteromedial nucleus (VPM) and the ventral posterolateral nucleus (VPL). In some species, the ventral posterolateral nucleus, pars caudalis is also a part of the VB. The VB gets inputs from the spinothalamic tract, medial lemniscus, and corticothalamic tract. The main output of the VB is the primary somatosensory cortex.

Frisson, also known as aesthetic chills or psychogenic shivers, is a psychophysiological response to rewarding stimuli that often induces a pleasurable or otherwise positively-valenced affective state and transient paresthesia, sometimes along with piloerection and mydriasis . The sensation commonly occurs as a mildly to moderately pleasurable emotional response to music with skin tingling; piloerection and pupil dilation not necessarily occurring in all cases.
The primary gustatory cortex (GC) is a brain structure responsible for the perception of taste. It consists of two substructures: the anterior insula on the insular lobe and the frontal operculum on the inferior frontal gyrus of the frontal lobe. Because of its composition the primary gustatory cortex is sometimes referred to in literature as the AI/FO(Anterior Insula/Frontal Operculum). By using extracellular unit recording techniques, scientists have elucidated that neurons in the AI/FO respond to sweetness, saltiness, bitterness, and sourness, and they code the intensity of the taste stimulus.

The wide dynamic range (WDR) neuron was first discovered by Mendell in 1966. Early studies of this neuron established what is known as the gate control theory of pain. The basic concept is that non-painful stimuli block the pathways for painful stimuli, inhibiting possible painful responses. This theory was supported by the fact that WDR neurons are responsible for responses to both painful and non-painful stimuli, and the idea that these neurons could not produce more than one of these responses simultaneously. WDR neurons respond to all types of somatosensory stimuli, make up the majority of the neurons found in the posterior grey column, and have the ability to produce long range responses including those responsible for pain and itch.
Somatosensory evoked potential is the electrical activity of the brain that results from the stimulation of touch. SEP tests measure that activity and are a useful, noninvasive means of assessing somatosensory system functioning. By combining SEP recordings at different levels of the somatosensory pathways, it is possible to assess the transmission of the afferent volley from the periphery up to the cortex. SEP components include a series of positive and negative deflections that can be elicited by virtually any sensory stimuli. For example, SEPs can be obtained in response to a brief mechanical impact on the fingertip or to air puffs. However, SEPs are most commonly elicited by bipolar transcutaneous electrical stimulation applied on the skin over the trajectory of peripheral nerves of the upper limb or lower limb, and then recorded from the scalp. In general, somatosensory stimuli evoke early cortical components, generated in the contralateral primary somatosensory cortex (S1), related to the processing of the physical stimulus attributes. About 100 ms after stimulus application, additional cortical regions are activated, such as the secondary somatosensory cortex (S2), and the posterior parietal and frontal cortices, marked by a parietal P100 and bilateral frontal N140. SEPs are routinely used in neurology today to confirm and localize sensory abnormalities, to identify silent lesions and to monitor changes during surgical procedures.
The parabrachial nuclei, also known as the parabrachial complex, are a group of nuclei in the dorsolateral pons that surrounds the superior cerebellar peduncle as it enters the brainstem from the cerebellum. They are named from the Latin term for the superior cerebellar peduncle, the brachium conjunctivum. In the human brain, the expansion of the superior cerebellar peduncle expands the parabrachial nuclei, which form a thin strip of grey matter over most of the peduncle. The parabrachial nuclei are typically divided along the lines suggested by Baxter and Olszewski in humans, into a medial parabrachial nucleus and lateral parabrachial nucleus. These have in turn been subdivided into a dozen subnuclei: the superior, dorsal, ventral, internal, external and extreme lateral subnuclei; the lateral crescent and subparabrachial nucleus along the ventrolateral margin of the lateral parabrachial complex; and the medial and external medial subnuclei

Mindfulness has been defined in modern psychological terms as "paying attention to relevant aspects of experience in a nonjudgmental manner", and maintaining attention on present moment experience with an attitude of openness and acceptance. Meditation is a platform used to achieve mindfulness. Both practices, mindfulness and meditation, have been "directly inspired from the Buddhist tradition" and have been widely promoted by Jon Kabat-Zinn. Mindfulness meditation has been shown to have a positive impact on several psychiatric problems such as depression and therefore has formed the basis of mindfulness programs such as mindfulness-based cognitive therapy, mindfulness-based stress reduction and mindfulness-based pain management. The applications of mindfulness meditation are well established, however the mechanisms that underlie this practice are yet to be fully understood. Many tests and studies on soldiers with PTSD have shown tremendous positive results in decreasing stress levels and being able to cope with problems of the past, paving the way for more tests and studies to normalize and accept mindful based meditation and research, not only for soldiers with PTSD, but numerous mental inabilities or disabilities.

Interoception is the collection of senses providing information to the organism about the internal state of the body. This can be both conscious and subconscious. It encompasses the brain's process of integrating signals relayed from the body into specific subregions—like the brainstem, thalamus, insula, somatosensory, and anterior cingulate cortex—allowing for a nuanced representation of the physiological state of the body. This is important for maintaining homeostatic conditions in the body and, potentially, facilitating self-awareness.
References
- ↑ "John B. Pierce Laboratory". Archived from the original on 2012-05-25. Retrieved 2016-12-15.
- ↑ Purves, Dale; Augustine, George J; Fitzpatrick, David; Katz, Lawrence C; LaMantia, Anthony-Samuel; McNamara, James O; Williams, S Mark (2001). "Central Pain Pathways: The Spinothalamic Tract". Sinauer Associates. Retrieved 2024-06-26.
- ↑ Baldwin, James Mark (1893-01-01). Elements of psychology. H. Holt and company. p. 103.
affective sensation.
- ↑ Douglas Olsen, G.; Pracejus, John W. (2004-01-01). "Integration of Positive and Negative Affective Stimuli". Journal of Consumer Psychology. 14 (4): 374–384. doi:10.1207/s15327663jcp1404_7.
- ↑ Williams, Marie; Cafarella, Paul; Olds, Timothy; Petkov, John; Frith, Peter (2010-08-01). "Affective descriptors of the sensation of breathlessness are more highly associated with severity of impairment than physical descriptors in people with COPD". Chest. 138 (2): 315–322. doi:10.1378/chest.09-2498. ISSN 1931-3543. PMID 20202942.
- ↑ Hall, G. B. C.; Kamath, M. V.; Collins, S.; Ganguli, S.; Spaziani, R.; Miranda, K. L.; Bayati, A.; Bienenstock, J. (2010-03-01). "Heightened central affective response to visceral sensations of pain and discomfort in IBS". Neurogastroenterology & Motility. 22 (3): 276–e80. doi:10.1111/j.1365-2982.2009.01436.x. ISSN 1365-2982. PMID 20003075. S2CID 19045986.
- ↑ Nolden, Alissa A.; Hayes, John E. (1 January 2017). "Perceptual and affective responses to sampled capsaicin differ by reported intake". Food Quality and Preference. 55: 26–34. doi:10.1016/j.foodqual.2016.08.003. PMC 5383096 . PMID 28392628.
- ↑ Grabenhorst, Fabian; Rolls, Edmund T.; Bilderbeck, Amy (2008-07-01). "How Cognition Modulates Affective Responses to Taste and Flavor: Top-down Influences on the Orbitofrontal and Pregenual Cingulate Cortices". Cerebral Cortex. 18 (7): 1549–1559. doi: 10.1093/cercor/bhm185 . ISSN 1047-3211. PMID 18056086.
- ↑ Rudenga, K.; Green, B.; Nachtigal, D.; Small, D. M. (2010-10-01). "Evidence for an Integrated Oral Sensory Module in the Human Anterior Ventral Insula". Chemical Senses. 35 (8): 693–703. doi:10.1093/chemse/bjq068. ISSN 0379-864X. PMC 2943409 . PMID 20595201.