
Muscimol is one of the principal psychoactive constituents of Amanita muscaria and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptor and displays sedative-hypnotic, depressant and hallucinogenic psychoactivity. This colorless or white solid is classified as an isoxazole.

Gut microbiota, gut microbiome, or gut flora, are the microorganisms, including bacteria, archaea, fungi, and viruses, that live in the digestive tracts of animals. The gastrointestinal metagenome is the aggregate of all the genomes of the gut microbiota. The gut is the main location of the human microbiome. The gut microbiota has broad impacts, including effects on colonization, resistance to pathogens, maintaining the intestinal epithelium, metabolizing dietary and pharmaceutical compounds, controlling immune function, and even behavior through the gut–brain axis.

Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Most stilbenoids are produced by plants, and the only known exception is the antihelminthic and antimicrobial stilbenoid, 2-isopropyl-5-[(E)-2-phenylvinyl]benzene-1,3-diol, biosynthesized by the Gram-negative bacterium Photorhabdus luminescens.

Hydrobiidae, commonly known as mud snails, is a large cosmopolitan family of very small freshwater and brackish water snails with an operculum; they are in the order Littorinimorpha.
Penicillium griseofulvum is a species of the genus of Penicillium which produces patulin, penifulvin A, cyclopiazonic acid, roquefortine C, shikimic acid, griseofulvin, and 6-Methylsalicylic acid. Penicillium griseofulvum occurs on cereals and nuts.

Chemical defense is a strategy employed by many organisms to avoid consumption by producing toxic or repellent metabolites or chemical warnings which incite defensive behavioral changes. The production of defensive chemicals occurs in plants, fungi, and bacteria, as well as invertebrate and vertebrate animals. The class of chemicals produced by organisms that are considered defensive may be considered in a strict sense to only apply to those aiding an organism in escaping herbivory or predation. However, the distinction between types of chemical interaction is subjective and defensive chemicals may also be considered to protect against reduced fitness by pests, parasites, and competitors. Repellent rather than toxic metabolites are allomones, a sub category signaling metabolites known as semiochemicals. Many chemicals used for defensive purposes are secondary metabolites derived from primary metabolites which serve a physiological purpose in the organism. Secondary metabolites produced by plants are consumed and sequestered by a variety of arthropods and, in turn, toxins found in some amphibians, snakes, and even birds can be traced back to arthropod prey. There are a variety of special cases for considering mammalian antipredatory adaptations as chemical defenses as well.
Penicillium decaturense is a species of the genus of Penicillium which was isolated from a fungus in North America. Penicillium decaturense produces citrinin, 15-Deoxyoxalicine B, decaturins A and decaturins A
Penicillium sclerotigenum is a species of fungus in the genus Penicillium. Described as new to science in 1955, it was first isolated from tubers of Chinese yam found in Japan. It is also associated with blue mold of yam in Korea. A DNA biosensor method for detecting the fungus in yam has been reported. The anti-insect compound sclerotigenin was reported from the fungus in 1999.
Penicillium raistrickii is an anamorph species of fungus in the genus Penicillium which produces griseofulvin, patulin and verruculogen.
Penicillium thiersii is a species of fungus in the genus Penicillium which was isolated from a wood decay fungi (Hypoxylon) in Wisconsin in North America. Penicillium thiersii produces thiersindole A, thiersindole B, thiersindole C, oxalicine A and oxalicine B

Desmethylflunitrazepam (also known as norflunitrazepam, Ro05-4435 and fonazepam) is a benzodiazepine that is a metabolite of flunitrazepam and has been sold online as a designer drug. It has an IC50 value of 1.499 nM for the GABAA receptor.
Aspergillus leporis is an anamorph species of fungus in the genus Aspergillus. It is from the Flavi section. The species was first described in 1979. It has been isolated from the dung of Lepus townsendii. Aspergillus leporis produces leporin A and leporin B. It has also been reported to produce antibiotic Y, kojic acid, and pseurotin.
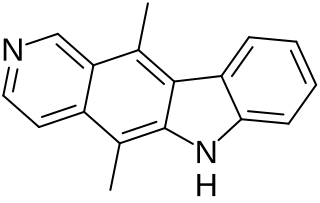
Ellipticine is an alkaloid first extracted from trees of the species Ochrosia elliptica and Rauvolfia sandwicensis, which inhibits the enzyme topoisomerase II via intercalative binding to DNA.

Stenocarpella maydis (Berk.) Sutton is a plant pathogenic fungus and causal organism of diplodia ear and stalk rot. Corn and canes are the only known hosts to date. No teleomorph of the fungus is known.

Corn sauce or fermented corn sauce is produced by fermentation using corn starch as the primary substrate. It is used as a food condiment and ingredient, both in paste and in powder form. Corn sauce, like soy sauce, has a characteristic savory taste. It is used to flavor dishes including soups, broths, and gravies.

Aszonalenin is an alkaloid which is produced by Neosartorya and Aspergillus species. Aszonalenin is a neurotoxin.
John Russell “Camille” Falck is an American chemist, Professor of Biochemistry, and holder of the Robert A. Welch Distinguish Chair in Chemistry at the University of Texas Southwestern Medical Center. In 1996 he was awarded the Wilfred T. Doherty Recognition Award from the Dallas-Forth Worth Section of the American Chemical Society and a Recognition Award at the March 10, 2002, Winter Eicosanoid Conference in appreciation of his significant contributions to the chemistry of natural products, and to the identification and functional characterization of the cytochrome P450 (P450) arachidonic acid (AA) monooxygenase metabolic pathway and its metabolites.










