Related Research Articles

T cells are one of the important types of white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface.

The T helper cells (Th cells), also known as CD4+ cells or CD4-positive cells, are a type of T cell that play an important role in the adaptive immune system. They aid the activity of other immune cells by releasing cytokines. They are considered essential in B cell antibody class switching, breaking cross-tolerance in dendritic cells, in the activation and growth of cytotoxic T cells, and in maximizing bactericidal activity of phagocytes such as macrophages and neutrophils. CD4+ cells are mature Th cells that express the surface protein CD4. Genetic variation in regulatory elements expressed by CD4+ cells determines susceptibility to a broad class of autoimmune diseases.
The regulatory T cells (Tregs or Treg cells), formerly known as suppressor T cells, are a subpopulation of T cells that modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease. Treg cells are immunosuppressive and generally suppress or downregulate induction and proliferation of effector T cells. Treg cells express the biomarkers CD4, FOXP3, and CD25 and are thought to be derived from the same lineage as naïve CD4+ cells. Because effector T cells also express CD4 and CD25, Treg cells are very difficult to effectively discern from effector CD4+, making them difficult to study. Research has found that the cytokine transforming growth factor beta (TGF-β) is essential for Treg cells to differentiate from naïve CD4+ cells and is important in maintaining Treg cell homeostasis.

FOXP3, also known as scurfin, is a protein involved in immune system responses. A member of the FOX protein family, FOXP3 appears to function as a master regulator of the regulatory pathway in the development and function of regulatory T cells. Regulatory T cells generally turn the immune response down. In cancer, an excess of regulatory T cell activity can prevent the immune system from destroying cancer cells. In autoimmune disease, a deficiency of regulatory T cell activity can allow other autoimmune cells to attack the body's own tissues.
In immunology, central tolerance is the process of eliminating any developing T or B lymphocytes that are autoreactive, i.e. reactive to the body itself. Through elimination of autoreactive lymphocytes, tolerance ensures that the immune system does not attack self peptides. Lymphocyte maturation occurs in primary lymphoid organs such as the bone marrow and the thymus. In mammals, B cells mature in the bone marrow and T cells mature in the thymus.
A thymocyte is an immune cell present in the thymus, before it undergoes transformation into a T cell. Thymocytes are produced as stem cells in the bone marrow and reach the thymus via the blood.
MHC-restricted antigen recognition, or MHC restriction, refers to the fact that a T cell can interact with a self-major histocompatibility complex molecule and a foreign peptide bound to it, but will only respond to the antigen when it is bound to a particular MHC molecule.

Hassall's corpuscles (or thymic corpuscles (bodies)) are structures found in the medulla of the human thymus, formed from eosinophilic type VI epithelial reticular cells arranged concentrically. These concentric corpuscles are composed of a central mass, consisting of one or more granular cells, and of a capsule formed of epithelioid cells. They vary in size with diameters from 20 to more than 100μm, and tend to grow larger with age. They can be spherical or ovoid and their epithelial cells contain keratohyalin and bundles of cytoplasmic fibres. Later studies indicate that Hassall's corpuscles differentiate from medullary thymic epithelial cells after they lose autoimmune regulator (AIRE) expression. This makes them an example of Thymic mimetic cells. They are named for Arthur Hill Hassall, who discovered them in 1846.
In immunology, peripheral tolerance is the second branch of immunological tolerance, after central tolerance. It takes place in the immune periphery. Its main purpose is to ensure that self-reactive T and B cells which escaped central tolerance do not cause autoimmune disease. Peripheral tolerance prevents immune response to harmless food antigens and allergens, too.

Harald von Boehmer was a German-Swiss immunologist best known for his work on T cells.
In immunology, clonal deletion is the removal through apoptosis of B cells and T cells that have expressed receptors for self before developing into fully immunocompetent lymphocytes. This prevents recognition and destruction of self host cells, making it a type of negative selection or central tolerance. Central tolerance prevents B and T lymphocytes from reacting to self. Thus, clonal deletion can help protect individuals against autoimmunity. Clonal deletion is thought to be the most common type of negative selection. It is one method of immune tolerance.
T-cell receptor revision is a process in the peripheral immune system which is used by mature T cells to alter their original antigenic specificity based on rearranged T cell receptors (TCR). This process can lead either to continuous appearance of potentially self-reactive T cells in the body, not controlled by the central tolerance mechanism in the thymus or better eliminate such self-reactive T cells on the other hand and thus contributing to peripheral tolerance - the extent of each has not been completely understood yet. This process occurs during follicular helper T cell formation in lymph node germinal centers.
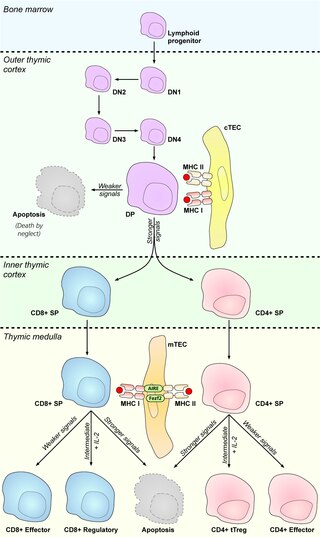
Medullary thymic epithelial cells (mTECs) represent a unique stromal cell population of the thymus which plays an essential role in the establishment of central tolerance. Therefore, mTECs rank among cells relevant for the development of functional mammal immune system.
Antigen transfer in the thymus is the transmission of self-antigens between thymic antigen-presenting cells which contributes to the establishment of T cell central tolerance.

Cortical thymic epithelial cells (cTECs) form unique parenchyma cell population of the thymus which critically contribute to the development of T cells.
Thymic epithelial cells (TECs) are specialized cells with high degree of anatomic, phenotypic and functional heterogeneity that are located in the outer layer (epithelium) of the thymic stroma. The thymus, as a primary lymphoid organ, mediates T cell development and maturation. The thymic microenvironment is established by TEC network filled with thymocytes in different developing stages. TECs and thymocytes are the most important components in the thymus, that are necessary for production of functionally competent T lymphocytes and self tolerance. Dysfunction of TECs causes several immunodeficiencies and autoimmune diseases.
Promiscuous gene expression (PGE), formerly referred to as ectopic expression, is a process specific to the thymus that plays a pivotal role in the establishment of central tolerance. This phenomenon enables generation of self-antigens, so called tissue-restricted antigens (TRAs), which are in the body expressed only by one or few specific tissues. These antigens are represented for example by insulin from the pancreas or defensins from the gastrointestinal tract. Antigen-presenting cells (APCs) of the thymus, namely medullary thymic epithelial cells (mTECs), dendritic cells (DCs) and B cells are capable to present peptides derived from TRAs to developing T cells and hereby test, whether their T cell receptors (TCRs) engage self entities and therefore their occurrence in the body can potentially lead to the development of autoimmune disease. In that case, thymic APCs either induce apoptosis in these autoreactive T cells or they deviate them to become T regulatory cells, which suppress self-reactive T cells in the body that escaped negative selection in the thymus. Thus, PGE is crucial for tissues protection against autoimmunity.
Thymoproteasome is a special kind of proteasome, which is present in vertebrates. In the body it is located in thymus, exclusively in cortical thymic epithelial cells (cTECs). But in thymus we can also find another type of specific proteasome, immunoproteasome, which is present in thymocytes, dendritic cells and medular thymic epithelial cells. It was first described in 2007 during a search for non-intronic sequence proximal to PSMB5 locus in mouse genome. The PSMB5 locus encodes the standard β5 proteasome subunit, while this sequence encodes a variant subunit β5t (PSMB11) specific to thymoproteasome. The importance of this protein complex is its involvement in positive selection of T cells.
Thymus stromal cells are subsets of specialized cells located in different areas of the thymus. They include all non-T-lineage cells, such as thymic epithelial cells (TECs), endothelial cells, mesenchymal cells, dendritic cells, and B lymphocytes, and provide signals essential for thymocyte development and the homeostasis of the thymic stroma.
Thymic mimetic cells are a heterogeneous population of cells located in the thymus that exhibit phenotypes of a wide variety of differentiated peripheral cells. They arise from medullary thymic epithelial cells (mTECs) and also function in negative selection of self-reactive T cells.
References
- 1 2 "Alfred Singer, M.D. | Center for Cancer Research". ccr.cancer.gov. Retrieved 2016-12-06.
- ↑ Singer, A.; Hathcock, K. S.; Hodes, R. J. (1980-03-01). "Cellular and genetic control of antibody responses. VIII. MHC restricted recognition of accessory cells, not B cells, by parent-specific subpopulations of normal F1 T helper cells". Journal of Immunology. 124 (3): 1079–1085. doi: 10.4049/jimmunol.124.3.1079 . ISSN 0022-1767. PMID 6153669. S2CID 25179621.
- 1 2 Singer, A.; Hathcock, K. S.; Hodes, R. J. (1982-01-01). "Self recognition in allogeneic thymic chimeras. Self recognition by T helper cells from thymus-engrafted nude mice is restricted to the thymic H-2 haplotype". The Journal of Experimental Medicine. 155 (1): 339–344. doi:10.1084/jem.155.1.339. ISSN 0022-1007. PMC 2186566 . PMID 6459401.
- 1 2 Van Laethem, François; Tikhonova, Anastasia N.; Pobezinsky, Leonid A.; Tai, Xuguang; Kimura, Motoko Y.; Le Saout, Cécile; Guinter, Terry I.; Adams, Anthony; Sharrow, Susan O. (2013-09-12). "Lck availability during thymic selection determines the recognition specificity of the T cell repertoire". Cell. 154 (6): 1326–1341. doi:10.1016/j.cell.2013.08.009. ISSN 1097-4172. PMC 3792650 . PMID 24034254.
- ↑ Mu, Jie; Tai, Xuguang; Iyer, Shankar S.; Weissman, Jocelyn D.; Singer, Alfred; Singer, Dinah S. (2014-03-15). "Regulation of MHC class I expression by Foxp3 and its effect on regulatory T cell function". Journal of Immunology. 192 (6): 2892–2903. doi:10.4049/jimmunol.1302847. ISSN 1550-6606. PMC 3952169 . PMID 24523508.
- ↑ Tai, Xuguang; Erman, Batu; Alag, Amala; Mu, Jie; Kimura, Motoko; Katz, Gil; Guinter, Terry; McCaughtry, Tom; Etzensperger, Ruth (2013-06-27). "Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals". Immunity. 38 (6): 1116–1128. doi:10.1016/j.immuni.2013.02.022. ISSN 1097-4180. PMC 3700677 . PMID 23746651.
- ↑ Kimura, Motoko Y.; Pobezinsky, Leonid A.; Guinter, Terry I.; Thomas, Julien; Adams, Anthony; Park, Jung-Hyun; Tai, Xuguang; Singer, Alfred (2013-02-01). "IL-7 signaling must be intermittent, not continuous, during CD8+ T cell homeostasis to promote cell survival instead of cell death". Nature Immunology. 14 (2): 143–151. doi:10.1038/ni.2494. ISSN 1529-2916. PMC 3552087 . PMID 23242416.
- ↑ Singer, Alfred; Adoro, Stanley; Park, Jung-Hyun (2008-10-01). "Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice". Nature Reviews Immunology. 8 (10): 788–801. doi:10.1038/nri2416. ISSN 1474-1733. PMC 2760737 . PMID 18802443.