
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.

Poison dart frog is the common name of a group of frogs in the family Dendrobatidae which are native to tropical Central and South America. These species are diurnal and often have brightly colored bodies. This bright coloration is correlated with the toxicity of the species, making them aposematic. Some species of the family Dendrobatidae exhibit extremely bright coloration along with high toxicity — a feature derived from their diet of ants, mites and termites— while species which eat a much larger variety of prey have cryptic coloration with minimal to no amount of observed toxicity. Many species of this family are threatened due to human infrastructure encroaching on their habitats.

Batrachotoxin (BTX) is an extremely potent cardiotoxic and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word βάτραχος, bátrachos, 'frog'. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.
Dihydroxylation is the process by which an alkene is converted into a vicinal diol. Although there are many routes to accomplish this oxidation, the most common and direct processes use a high-oxidation-state transition metal. The metal is often used as a catalyst, with some other stoichiometric oxidant present. In addition, other transition metals and non-transition metal methods have been developed and used to catalyze the reaction.

(+)-Discodermolide is a polyketide natural product found to stabilize microtubules. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources. The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.

Lupinine is a quinolizidine alkaloid present in the genus Lupinus of the flowering plant family Fabaceae. The scientific literature contains many reports on the isolation and synthesis of this compound as well as a vast number of studies on its biosynthesis from its natural precursor, lysine. Studies have shown that lupinine hydrochloride is a mildly toxic acetylcholinesterase inhibitor and that lupinine has an inhibitory effect on acetylcholine receptors. The characteristically bitter taste of lupin beans, which come from the seeds of Lupinus plants, is attributable to the quinolizidine alkaloids which they contain, rendering them unsuitable for human and animal consumption unless handled properly. However, because lupin beans have potential nutritional value due to their high protein content, efforts have been made to reduce their alkaloid content through the development of "sweet" varieties of Lupinus.

The strawberry poison frog, strawberry poison-dart frog or blue jeans poison frog is a species of small poison dart frog found in Central America. It is common throughout its range, which extends from eastern central Nicaragua through Costa Rica and northwestern Panamá. The species is often found in humid lowlands and premontane forest, but large populations are also found in disturbed areas such as plantations. The strawberry poison frog is perhaps most famous for its widespread variation in coloration, comprising approximately 15–30 color morphs, most of which are presumed to be true-breeding. O. pumilio, while not the most poisonous of the dendrobatids, is the most toxic member of its genus.

The dyeing poison dart frog, also known as the cobalt poison frog, tinc, or dyeing poison frog, is a species of poison dart frog. It is among the most variably colored and largest species of poison dart frogs, typically reaching snout–vent lengths of about 50 mm (2.0 in). It is distributed in the eastern portion of the Guiana Shield, including parts of French Guiana, Guyana, Suriname and Brazil.

Phyllobates is a genus of poison dart frogs native to Central and South America, from Nicaragua to Colombia. There are 3 different Colombian species of Phyllobates, considered highly toxic species due to the poison they contain in the wild.
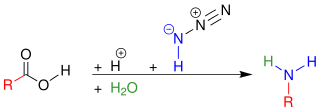
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. The intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products. The reaction is effective with carboxylic acids to give amines (above), and with ketones to give amides (below).

Pumiliotoxin 251D is a toxic organic compound. It is found in the skin of poison frogs from the genera Dendrobates, Epipedobates, Minyobates, and Phyllobates and toads from the genus Melanophryniscus. Its name comes from the pumiliotoxin family (PTXs) and its molecular mass of 251 daltons. When the toxin enters the bloodstream through cuts in the skin or by ingestion, it can cause hyperactivity, convulsions, cardiac arrest and ultimately death. It is especially toxic to arthropods, even at low concentrations.

Pumiliotoxins (PTXs), are one of several toxins found in the skin of poison dart frogs. The frog species, P. bibronii also produces PTXs to deter predators. Closely related, though more toxic, are allopumiliotoxins, (aPTXs). Other toxins found in the skin of poison frogs include decahydroquinolines (DHQs), izidines, coccinellines, and spiropyrrolizidine alkaloids. Pumiliotoxins are very poisonous in high concentrations. Pumiliotoxins are much weaker than batrachotoxins, ranging between 100 and 1000 times less poisonous. There are three different types of this toxin: A, B and C, of which toxins A and B are more toxic than C. Pumiliotoxins interfere with muscle contraction by affecting calcium channels, causing partial paralysis, difficulty moving, hyperactivity, or death. The median lethal dose of pumiliotoxins A and B is 50 μg / mouse, 20 μg / mouse respectively, while the amount of pumiliotoxin is 200 μg / frog.
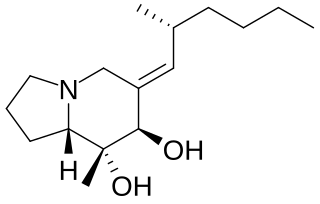
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates. It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D. It has been tested on mice and found to be five times more potent than the former version. It has been produced synthetically through a variety of different routes.
The Fleming–Tamao oxidation, or Tamao–Kumada–Fleming oxidation, converts a carbon–silicon bond to a carbon–oxygen bond with a peroxy acid or hydrogen peroxide. Fleming–Tamao oxidation refers to two slightly different conditions developed concurrently in the early 1980s by the Kohei Tamao and Ian Fleming research groups.

Deoxyepinephrine, also known by the common names N-methyldopamine and epinine, is an organic compound and natural product that is structurally related to the important neurotransmitters dopamine and epinephrine. All three of these compounds also belong to the catecholamine family. The pharmacology of epinine largely resembles that of its "parent", dopamine. Epinine has been found in plants, insects and animals. It is also of significance as the active metabolic breakdown product of the prodrug ibopamine, which has been used to treat congestive heart failure.

Histrionicotoxins are a group of related toxins found in the skin of poison frogs from the family Dendrobatidae, notably Oophaga histrionica, which are native to Colombia. It is likely that, as with other poison frog alkaloids, histrionicotoxins are not manufactured by the amphibians, but absorbed from insects in their diet and stored in glands in their skin. They are notably less toxic than other alkaloids found in poison frogs, yet their distinct structure acts as a neurotoxin by non-competitive inhibition of nicotinic acetylcholine receptors.

Adelphobates is a small genus of poison dart frogs. They are found in the central and lower Amazon basin of Peru and Brazil, possibly Bolivia. It was originally erected as a sister group to the Dendrobates and Oophaga genera. The validity of the genus is still being discussed, with the alternative being "Dendrobates galactonotus group" within Dendrobates. One species originally placed in this genus as Adelphobates captivus has since been moved to the genus Excidobates erected in 2008.
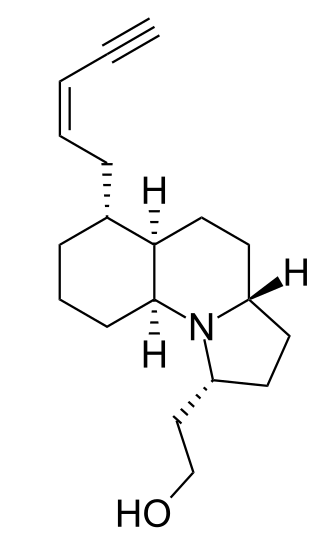
Gephyrotoxin is a naturally occurring product that stems from the Colombian tropical frog Dendrobates histrionicus. It is a member of the class of compounds known as histrionicotoxins. This alkaloid skin secretion was first isolated from the tropical frog in 1977 by Daly and his fellow workers.

Phantasmidine is a toxic substance derived from the Ecuadorian poisonous frog Anthony's poison arrow frog, more commonly known as the “phantasmal poison frog”. It is a nicotinic agonist, meaning it binds to nicotinic receptors in the body and mimics the effects of the neurotransmitter acetylcholine. This causes the stimulation of the body's parasympathetic nervous system, which induces many inhibitory behaviors in the body such as decreased heart rate.


















