A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words lactone + amide.
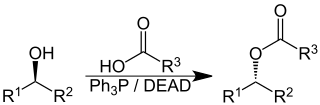
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). Typical protocol is to add the phosphine and azodicarboxylate together at -10C, typically in THF or toluene, until a white precipitate forms. This white, cloudy suspension is the ylide. Then a solution of the nucleophile and alcohol are added together and the reaction can, and in many cases is, heated to reflux. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion as the nucleophile displaces it. A common side-product is produced when the azodicarboxylate displaces the leaving group instead of the desired nucleophile. This happens if the nucleophile is not acidic enough or is not nucleophilic enough due to steric or electronic constraints. A variation of this reaction utilizing a nitrogen nucleophile is known as a Fukuyama-Mitsunobu.
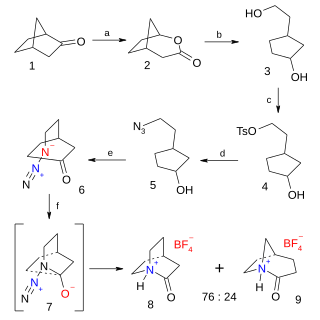
Quinuclidones are a class of bicyclic organic compounds with chemical formula C7H11NO with two structural isomers for the base skeleton 3-quinuclidone and 2-quinuclidone.
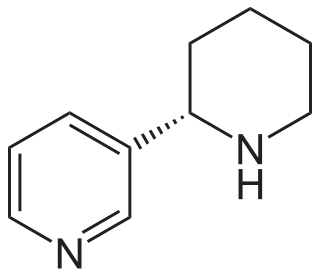
Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco plant, a close relative of the common tobacco plant. It is a structural isomer of, and chemically similar to, nicotine. Its principal (historical) industrial use is as an insecticide.

The Wenker synthesis is an organic reaction converting a beta amino alcohol to an aziridine with the help of sulfuric acid. It is used industrially for the synthesis of aziridine itself.
The Kulinkovich reaction describes the organic synthesis of cyclopropanols via reaction of esters with dialkyldialkoxytitanium reagents, generated in situ from Grignard reagents bearing hydrogen in beta-position and titanium(IV) alkoxides such as titanium isopropoxide. This reaction was first reported by Oleg Kulinkovich and coworkers in 1989.
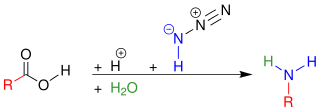
The Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually a aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophenone and hydrazoic acid to benzanilide. Surprisingly, the intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural products.
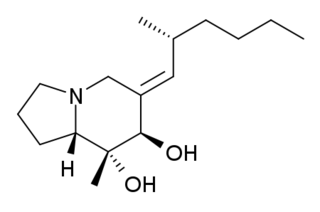
Allopumiliotoxin 267A is a toxin found in the skin of several poison frogs of the family Dendrobates. It is a member of the class of compounds known as allopumiliotoxins. The frogs produce the toxin by modifying the original version, pumiliotoxin 251D. It has been tested on mice and found to be five times more potent than the former version. It has been produced synthetically through a variety of different routes.
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula CH3SO2Cl. Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group CH3SO2, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene (methylenedioxosulfur(VI)).

Epiboxidine is a chemical compound which acts as a partial agonist at neural nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes. It was developed as a less toxic analogue of the potent frog-derived alkaloid epibatidine, which is around 200 times stronger than morphine as an analgesic but produces extremely dangerous toxic nicotinic side effects.

3β-(p-Fluorobenzoyloxy)tropane, is a tropane derivative drug which acts as a local anaesthetic, having around 30% the stimulant potency of cocaine but around the same potency as a local anaesthetic. It has been investigated as a potential radiolabelled agent for studying receptor binding, but was not adopted for this application. The main application for fluorotropacocaine, however, has been as a designer drug analogue of cocaine, first detected by the EMCDDA in 2008, and subsequently sold as an ingredient of various "bath salt" powder products, usually mixed in combination with other stimulant drugs such as caffeine, dimethocaine, desoxypipradrol or substituted cathinone derivatives.

Indolizine(Chemical formula C8H7N) is a heterocyclic aromatic organic compound that is an isomer of indole. The saturated analog indolizidine forms the structural core of a variety of alkaloids such as swainsonine.

Morphan is a chemical compound.

AR-R17779 is a drug that acts as a potent and selective full agonist for the α7 subtype of neural nicotinic acetylcholine receptors. It has nootropic effects in animal studies, but its effects do not substitute for those of nicotine. It has also been studied as a potential novel treatment for arthritis.
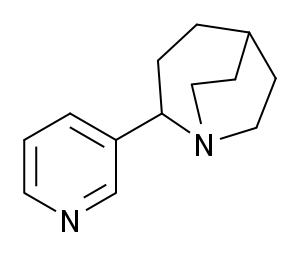
TC-1698 is a drug developed by Targacept which acts as a partial agonist for the α7 subtype of neural nicotinic acetylcholine receptors. It has neuroprotective effects in animal studies, and has been used as a lead compound to find further potent derivatives.
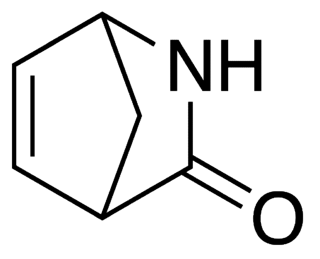
Vince lactam is the commercial name given to the bicyclic molecule γ-lactam 2-azabicyclo[2.2.1]hept-5-en-3-one. This lactam is a versatile chemical intermediate used in organic and medicinal chemistry. It is used as a synthetic precursor for three drugs. It is named after Robert Vince who has used the structural features of this molecule for the preparation of carbocyclic nucleosides. Vince's work with this lactam eventually led to his synthesis of abacavir. Peramivir synthesis is also dependent on Vince lactam starting material.

The Enders SAMP/RAMP hydrazone alkylation reaction is an asymmetric carbon-carbon bond formation reaction facilitated by pyrrolidine chiral auxiliaries. It was pioneered by E. J. Corey and D. Enders in 1976, and was further developed by D. Enders and his group. This method is usually a three-step sequence. The first step is to form the hydrazone between (S)-1-amino-2-methoxymethylpyrrolidine (SAMP) or (R)-1-amino-2-methoxymethylpyrrolidine (RAMP) and a ketone or aldehyde. Afterwards, the hydrazone is deprotonated by lithium diisopropylamide (LDA) to form an azaenolate, which reacts with alkyl halides or other suitable electrophiles to give alkylated hydrazone species with the simultaneous generation of a new chiral center. Finally, the alkylated ketone or aldehyde can be regenerated by ozonolysis or hydrolysis.

RJR-2429 is a drug that acts as an agonist at neural nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes. RJR-2429 is stronger than nicotine but weaker than epibatidine in most assays, and with high affinity for both α3β4 and α4β2 subtypes, as well as the less studied α1βγδ subtype.
The Blum–Ittah aziridine synthesis, also known as the Blum–Ittah-Shahak aziridine synthesis or simply the Blum aziridine synthesis is a name reaction of organic chemistry, for the generation of aziridines from oxiranes.















