
Abscisic acid is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.

Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates.

Harmaline is a fluorescent indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partly hydrogenated form of harmine.

Castanospermum is a monotypic genus in the legume family Fabaceae. The sole species is Castanospermum australe, commonly known as Moreton Bay chestnut or black bean, which is native to rainforested areas on the east coast of Queensland and northeastern New South Wales, and to the southwest Pacific islands of Vanuatu and New Caledonia

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Umifenovir, sold under the brand name Arbidol, is sold and used as an antiviral medication for influenza in Russia and China. The drug is manufactured by Pharmstandard. It is not approved by the U.S. Food and Drug Administration (FDA) for the treatment or prevention of influenza.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.

Swainsonine is an indolizidine alkaloid. It is a potent inhibitor of Golgi alpha-mannosidase II, an immunomodulator, and a potential chemotherapy drug. As a toxin in locoweed it also is a significant cause of economic losses in livestock industries, particularly in North America. It was first isolated from Swainsona canescens.
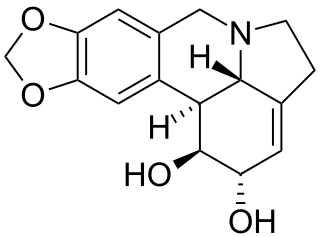
Lycorine is a toxic crystalline alkaloid found in various Amaryllidaceae species, such as the cultivated bush lily, surprise lilies (Lycoris), and daffodils (Narcissus). It may be highly poisonous, or even lethal, when ingested in certain quantities. Regardless, it is sometimes used medicinally, a reason why some groups may harvest the very popular Clivia miniata.

Pyrrolizidine alkaloids (PAs), sometimes referred to as necine bases, are a group of naturally occurring alkaloids based on the structure of pyrrolizidine. Their use dates back centuries and is intertwined with the discovery, understanding, and eventual recognition of their toxicity on humans and animals.

Griffithsin is a protein isolated from the red algae Griffithsia. It has a 121-amino acid sequence which exhibits a Jacalin-like lectin fold. Several structures of this protein have been solved by X-ray crystallography and deposited in the PDB. It has been shown in vitro to be a highly potent HIV entry inhibitor. It is currently being investigated as a potential microbicide for use in the prevention of the transmission of HIV.
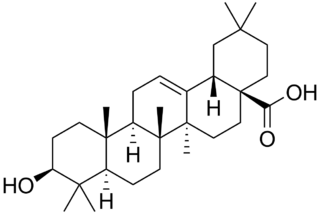
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins.

Aurintricarboxylic acid (ATA) is a chemical compound that readily polymerizes in aqueous solution, forming a stable free radical that inhibits protein-nucleic acid interactions. It is a potent inhibitor of ribonuclease and topoisomerase II by preventing the binding of the nucleic acid to the enzyme. It stimulates tyrosine phosphorylation processes including the Jak2/STAT5 pathway in NB2 lymphoma cells, ErbB4 in neuroblastoma cells, and MAP kinases, Shc proteins, phosphatidylinositide 3-kinase and phospholipase Cγ in PC12 cells. It also inhibits apoptosis. It prevents down-regulation of Ca2+-impermeable GluR2 receptors and inhibits calpain, a Ca2+-activated protease that is activated during apoptosis.

Radical S-adenosyl methionine domain-containing protein 2 is a protein that in humans is encoded by the RSAD2 gene. RSAD2 is a multifunctional protein in viral processes that is an interferon stimulated gene. It has been reported that viperin could be induced by either IFN-dependent or IFN-independent pathways and certain viruses may use viperin to increase their infectivity.

Simeprevir, sold under the brand name Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.

Nonstructural protein 5A (NS5A) inhibitors are direct acting antiviral agents (DAAs) that target viral proteins, and their development was a culmination of increased understanding of the viral life cycle combined with advances in drug discovery technology. However, their mechanism of action is complex and not fully understood. NS5A inhibitors were the focus of much attention when they emerged as a part of the first curative treatment for hepatitis C virus (HCV) infections in 2014. Favorable characteristics have been introduced through varied structural changes, and structural similarities between NS5A inhibitors that are clinically approved are readily apparent. Despite the recent introduction of numerous new antiviral drugs, resistance is still a concern and these inhibitors are therefore always used in combination with other drugs.
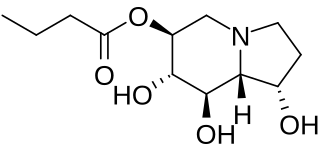
Celgosivir, in development by Migenix for the treatment of hepatitis C virus (HCV) infection, is an oral prodrug of the natural product castanospermine that inhibits alpha-glucosidase I, an enzyme that plays a critical role in viral maturation by initiating the processing of the N-linked oligosaccharides of viral envelope glycoproteins. Celgosivir is well absorbed in vitro and in vivo, and is rapidly converted to castanospermine. Celgosivir has a novel mechanism of action, and demonstrates broad antiviral activity in vitro.

Interferon lambda 4 is one of the most recently discovered human genes and the newest addition to the interferon lambda protein family. This gene encodes the IFNL4 protein, which is involved in immune response to viral infection.
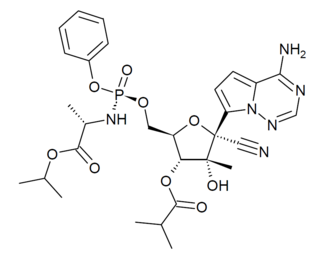
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing, it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable. It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.
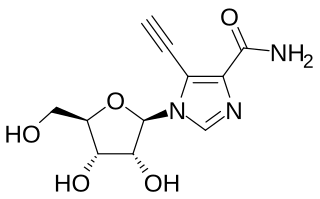
EICAR (5-ethynyl-1-β-D-ribofuranosylimidazole-4-carboxamide) is a nucleoside analogue which has both anti-cancer and antiviral effects, and was originally developed for the treatment of leukemia, but was unsuccessful in human clinical trials. It has broad spectrum antiviral effects with activity against pox viruses, Semliki forest virus, Junin virus, reovirus, influenza, measles virus and respiratory syncytial virus among others, although it is not active against coronaviridae such as SARS-CoV-1. This useful spectrum of activity means that EICAR and related derivatives continue to be investigated for the treatment of viral diseases.






















