
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium Micromonospora echinospora, with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs. It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML). A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Calicheamicin γ1 and the related enediyne esperamicin are the two of the most potent antitumor agents known.

Rebeccamycin (NSC 655649) is a weak topoisomerase I inhibitor isolated from Nocardia sp. It is structurally similar to staurosporine, but does not show any inhibitory activity against protein kinases. It shows significant antitumor properties in vitro (IC50=480nM against mouse B16 melanoma cells and IC50=500nM against P388 leukemia cells). It is an antineoplastic antibiotic and an intercalating agent.

Enediynes are organic compounds containing two triple bonds and one double bond.

Asterric acid is a fungal metabolite that can inhibit endothelin binding, first isolated from Aspergillus terreus. Its derivatives and similar phenolic fungal isolates are a subject of research on anti-angiogenic compounds.

Kidamycin is an anthracycline antibiotic with anticancer activity. It was first synthesized from a strain of streptomyces bacteria isolated from a soil sample. In clinical trials, Kindamycin showed high effect against gram positive bacteria as well as multiple cancer models including Ehrlich ascites carcinoma, Sarcoma 180, NF-sarcoma, and Yoshida sarcoma.
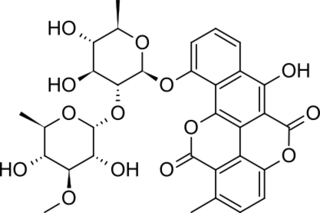
Chartreusin is an antibiotic originally isolated from the bacteria Streptomyces Chartreusis. The crystalline compound itself has a yellow-green colour, as per its name, and is stable at room temperature for several hours. Chartreusin is chemically related to elsamitrucin, as the two share an aglycone chartarin structure, though they differ in their sugar moieties. Both chartreusin and elsamitrucin were found to have anticancer activity.
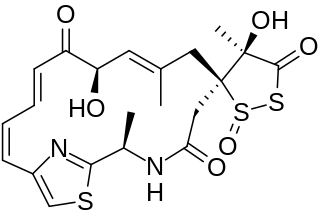
Leinamycin is an 18-membered macrolactam produced by several species of Streptomyces atroolivaceus. This macrolactam has also been shown to exhibit antitumor properties as well as antimicrobial properties against gram-positive and gram-negative bacteria. The presence of a spiro-fused 1,3-dioxo-1,2-dithiolane moiety was a unique structural property at the time of this compound's discovery and it plays an important role in leinamycin's antitumor and antibacterial properties due to its ability to inhibit DNA synthesis.
Micromonospora citrea is an endophytic actinomycete. It produces citreamicins, several types of antibacterial antibiotics.
Micromonospora sagamiensis is an endophytic actinomycete. It produces sagamicin, an aminoglycoside antibiotic, as well as several mutational variants. Its cell wall contains only D-alanine.

Herboxidiene is a polyketide molecule soluble in polar solvents such as water, ethanol, n-butanol and acetone but insoluble in non-polar molecule such as hexane. It was first isolated from the fermentation broth of Streptomyces chromofuscus by researchers in Monsanto Company in 1992. Herboxidiene shows in vitro antitumor activity by targeting the SF3B protein in the splicesosome. Many antitumor derivatives have also been developed from herboxidiene through chemical modification.

The arylomycins are a class of antibiotics initially isolated from a soil sample obtained in Cape Coast, Ghana. In this initial isolation, two families of closely related arylomycins, A and B, were identified. The family of glycosylated arylomycin C lipopeptides were subsequently isolated from a Streptomyces culture in a screen for inhibitors of bacterial signal peptidase. The initially isolated arylomycins have a limited spectrum of activity against Gram-positive bacteria, including Staphylococcus aureus and Streptococcus pneumoniae. The only activity against Gram-negative bacteria was seen in strains with a compromised outer membrane.
Gymnascella dankaliensis is a moderate to slow growing fungus commonly found in the soil of warmer climates. It is characterized by round yellow, orange or red-brown ascospores encircled by undifferentiated filaments. They have been found in ear, nail and skin infections. Their metabolites have been isolated and shown to have cytotoxic and anti-tumor properties.
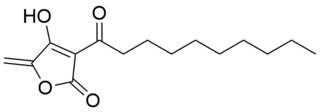
Agglomerins are bacterial natural products, identified as metabolites of Pantoea agglomerans which was isolated in 1989 from river water in Kobe, Japan. They belong to the class of tetronate antibiotics, which include tetronomycin, tetronasin, and abyssomicin C. The members of the agglomerins differ only in the composition of the acyl chain attached to the tetronate ring. They possess antibiotic activity against anaerobic bacteria and weak activity against aerobic bacteria in vitro. The structures were solved in 1990. Agglomerin A is the major component (38%), followed by agglomerin B (30%), agglomerin C (24%), and agglomerin D (8%).
Quinolidomicin A1 is a 60-membered macrocyclic compound isolated from Micromonospora sp. JY16. Quinolidomicins are a class of macrolides that contain a benzoquinone chromophore as well as an immense lactone ring, which far surpasses that in monozanomycin. It is currently the largest identified macrolide of terrestrial origin. It was initially discovered when in a screening for anti-tumor antibiotics, where it was found to be cytotoxic against P388 murine leukemia cells (IC50 8 nM), and has later been found to have strong cytotoxic activity against HT-29, MKN28, K562, and KB.

Naphthablin is a naphthoquinone compound with the molecular formula C29H36O8 which is produced by the bacterium Streptomyces aculeolatus.

Nagstatin is a strong competitive inhibitor of the N-acetyl-β-d-glucosaminidase with the molecular formula C12H17N3O6. Nagstatin is produced by the bacterium Streptomyces amakusaensis.
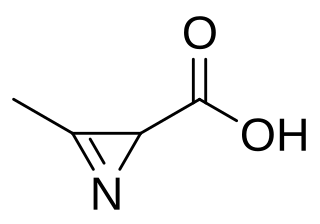
Azirinomycin is an antibiotic azirine derivative with the molecular formula C4H5NO2 which is produced by the bacterium Streptomyces aureus. Azirinomycin was first isolated in 1971. Azirinomycin is toxic and therefore it cannot not be used in human medicine.
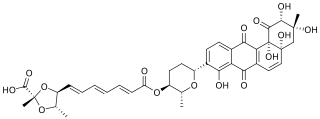
Dioxamycin is a benz[a] anthraquinone antibiotic and kinase inhibitor with the molecular formula C38H40O15. Dioxamycin is produced by the bacterium Streptomyces cocklensis and Streptomyces xantholiticus.
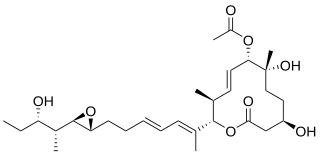
Pladienolide B is a natural product produced by bacterial strain, Streptomyces platensis MER-11107, which is a gram-positive bacteria isolated from soil in Japan. Pladienolide B is a molecule of interest due to its potential anti-cancer properties. Its anti-cancer mode of action includes binding to the SF3B complex in the U2 snRNP in the human spliceosome.

Disorazol, a cyclic polyketide synthesized by the bacterium Sorangium cellulosum So ce12, was first detected and isolated in 1994. Its chemical structure consists of a macrocyclic ring and two oxazole rings. Disorazol A has been demonstrated to exhibit anti-fungi activities, but it was not active against yeasts. In addition, this substance demonstrates potent anti-cancer characteristics at exceptionally low picomolar levels by obstructing the mechanism of tubulin assembly and triggering the disruption of microtubules. As a result, these impacts lead to the initiation of cell apoptosis. However, disorazols cannot be directly used as drugs in the clinic due to its extremely high cytotoxicity and instability. Thus, chemical and biosynthetic synthesis pathways were designed to synthesize unnatural derivatives of disorazol in hope of reducing its cytotoxicity without decreasing its anti-cancer potency.
















