Thermodynamic properties

I. cubic, II. hcp, III. fcc, IV. orthorhombic
| Phase behavior | |
|---|---|
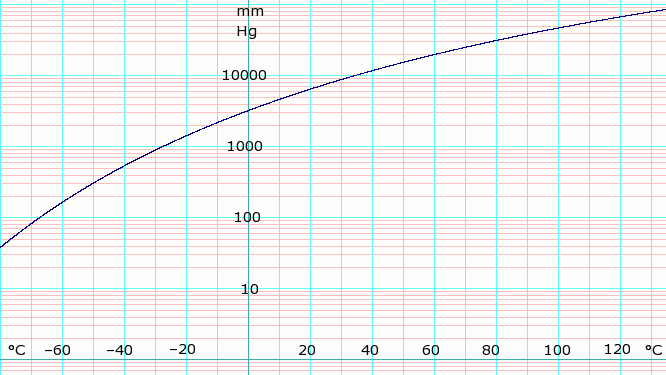
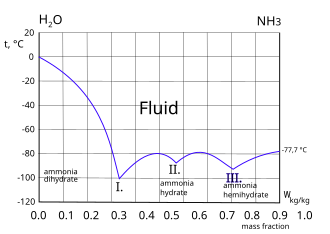
| Triple point | 195.4 K (−77.75 °C), 6.060 kPa |
| Critical point | 405.5 K (132.3 °C), 11.300 MPa |
| Std enthalpy change of fusion, ΔfusH | +5.653 kJ/mol |
| Std entropy change of fusion, ΔfusS | +28.93 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH | +23.35 kJ/mol at BP of −33.4 °C |
| Std entropy change of vaporization, ΔvapS | +97.41 J/(mol·K) at BP of −33.4 °C |
| Solid properties | |
| Std enthalpy change of formation, ΔfH | ? kJ/mol |
| Standard molar entropy, S | ? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH | −80.882 ± 0.053 kJ/mol [2] |
| Standard molar entropy, S | ? J/(mol K) |
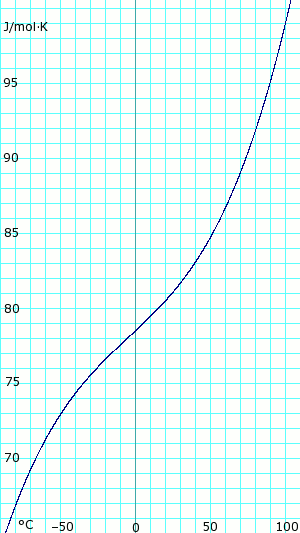
| Heat capacity, cp | 80.80 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH | −45.556 ± 0.029 kJ/mol [3] |
| Std Gibbs free energy change of formation, ΔfG | −16.6 kJ/mol |
| Standard molar entropy, S | 192.77 J/(mol K) |
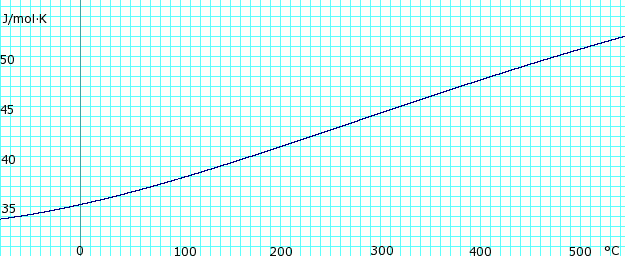
| Heat capacity, cp | 35.06 J/(mol K) |
| Heat capacity ratio, γ at 15 °C | 1.310 |
| van der Waals' constants | a = 422.5 L 2 kPa/mol 2 b = 0.03707 L/mol |