High-density lipoprotein (HDL) is one of the five major groups of lipoproteins. Lipoproteins are complex particles composed of multiple proteins which transport all fat molecules (lipids) around the body within the water outside cells. They are typically composed of 80–100 proteins per particle. HDL particles enlarge while circulating in the blood, aggregating more fat molecules and transporting up to hundreds of fat molecules per particle.

Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to cells. LDL is involved in atherosclerosis, a process in which it is oxidized within the walls of arteries.

Hypercholesterolemia, also called high cholesterol, is the presence of high levels of cholesterol in the blood. It is a form of hyperlipidemia, hyperlipoproteinemia, and dyslipidemia.

Chylomicrons, also known as ultra low-density lipoproteins (ULDL), are lipoprotein particles that consist of triglycerides (85–92%), phospholipids (6–12%), cholesterol (1–3%), and proteins (1–2%). They transport dietary lipids from the intestines to other locations in the body. ULDLs are one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream. A protein specific to chylomicrons is ApoB48.

Apolipoproteins are proteins that bind lipids to form lipoproteins. They transport lipids in blood, cerebrospinal fluid and lymph.
Hyperlipidemia is abnormally elevated levels of any or all lipids or lipoproteins in the blood. The term hyperlipidemia refers to the laboratory finding itself and is also used as an umbrella term covering any of various acquired or genetic disorders that result in that finding. Hyperlipidemia represents a subset of dyslipidemia and a superset of hypercholesterolemia. Hyperlipidemia is usually chronic and requires ongoing medication to control blood lipid levels.

The low-density lipoprotein receptor (LDL-R) is a mosaic protein of 839 amino acids that mediates the endocytosis of cholesterol-rich low-density lipoprotein (LDL). It is a cell-surface receptor that recognizes apolipoprotein B100 (ApoB100), which is embedded in the outer phospholipid layer of very low-density lipoprotein (VLDL), their remnants—i.e. intermediate-density lipoprotein (IDL), and LDL particles. The receptor also recognizes apolipoprotein E (ApoE) which is found in chylomicron remnants and IDL. In humans, the LDL receptor protein is encoded by the LDLR gene on chromosome 19. It belongs to the low density lipoprotein receptor gene family. It is most significantly expressed in bronchial epithelial cells and adrenal gland and cortex tissue.
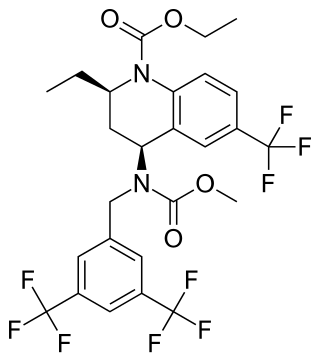
Torcetrapib was a drug being developed to treat hypercholesterolemia and prevent cardiovascular disease. Its development was halted in 2006 when phase III studies showed excessive all-cause mortality in the treatment group receiving a combination of atorvastatin (Lipitor) and torcetrapib.
Cholesteryl ester transfer protein (CETP), also called plasma lipid transfer protein, is a plasma protein that facilitates the transport of cholesteryl esters and triglycerides between the lipoproteins. It collects triglycerides from very-low-density (VLDL) or Chylomicrons and exchanges them for cholesteryl esters from high-density lipoproteins (HDL), and vice versa. Most of the time, however, CETP does a heteroexchange, trading a triglyceride for a cholesteryl ester or a cholesteryl ester for a triglyceride.
The lipid hypothesis is a medical theory postulating a link between blood cholesterol levels and the occurrence of cardiovascular disease. A summary from 1976 described it as: "measures used to lower the plasma lipids in patients with hyperlipidemia will lead to reductions in new events of coronary heart disease". It states, more concisely, that "decreasing blood cholesterol [...] significantly reduces coronary heart disease".

Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels of low-density lipoprotein cholesterol, in the blood and early cardiovascular diseases. The most common mutations diminish the number of functional LDL receptors in the liver or produce abnormal LDL receptors that never go to the cell surface to function properly. Since the underlying body biochemistry is slightly different in individuals with FH, their high cholesterol levels are less responsive to the kinds of cholesterol control methods which are usually more effective in people without FH. Nevertheless, treatment is usually effective.

Lipoprotein(a) is a low-density lipoprotein variant containing a protein called apolipoprotein(a). Genetic and epidemiological studies have identified lipoprotein(a) as a risk factor for atherosclerosis and related diseases, such as coronary heart disease and stroke.

Apolipoprotein AI(ApoA-I) is a protein that in humans is encoded by the APOA1 gene. As the major component of HDL particles, it has a specific role in lipid metabolism.

Apolipoprotein C-III also known as apo-CIII, and apolipoprotein C3, is a protein that in humans is encoded by the APOC3 gene. Apo-CIII is secreted by the liver as well as the small intestine, and is found on triglyceride-rich lipoproteins such as chylomicrons, very low density lipoprotein (VLDL), and remnant cholesterol.
Endothelial lipase (LIPG) is a form of lipase secreted by vascular endothelial cells in tissues with high metabolic rates and vascularization, such as the liver, lung, kidney, and thyroid gland. The LIPG enzyme is a vital component to many biological processes. These processes include lipoprotein metabolism, cytokine expression, and lipid composition in cells. Unlike the lipases that hydrolyze Triglycerides, endothelial lipase primarily hydrolyzes phospholipids. Due to the hydrolysis specificity, endothelial lipase contributes to multiple vital systems within the body. On the contrary to the beneficial roles that LIPG plays within the body, endothelial lipase is thought to play a potential role in cancer and inflammation. Knowledge obtained in vitro and in vivo suggest the relations to these conditions, but human interaction knowledge lacks due to the recent discovery of endothelial lipase. Endothelial lipase was first characterized in 1999. The two independent research groups which are notable for this discovery cloned the endothelial lipase gene and identified the novel lipase secreted from endothelial cells. The anti-Atherosclerosis opportunity through alleviating plaque blockage and prospective ability to raise High-density lipoprotein (HDL) have gained endothelial lipase recognition.

ATP-binding cassette transporter ABCA1, also known as the cholesterol efflux regulatory protein (CERP) is a protein which in humans is encoded by the ABCA1 gene. This transporter is a major regulator of cellular cholesterol and phospholipid homeostasis.

Apolipoprotein A-V is a protein that in humans is encoded by the APOA5 gene on chromosome 11. It is significantly expressed in liver. The protein encoded by this gene is an apolipoprotein and an important determinant of plasma triglyceride levels, a major risk factor for coronary artery disease. It is a component of several lipoprotein fractions including VLDL, HDL, chylomicrons. It is believed that apoA-V affects lipoprotein metabolism by interacting with LDL-R gene family receptors. Considering its association with lipoprotein levels, APOA5 is implicated in metabolic syndrome. The APOA5 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

Familial dysbetalipoproteinemia or type III hyperlipoproteinemia is a condition characterized by increased total cholesterol and triglyceride levels, and decreased HDL levels.
Steven E. Nissen is an American cardiologist, researcher and patient advocate. He was chairman of cardiovascular medicine at the Cleveland Clinic, in Cleveland, Ohio.
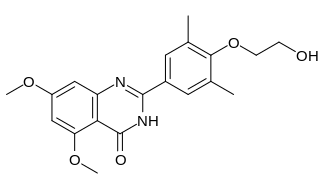
Apabetalone is an orally available small molecule created by Resverlogix Corp. that is being evaluated in clinical trials for the treatment of atherosclerosis and associated cardiovascular disease (CVD). In the phase II clinical trial ASSURE in patients with angiographic coronary disease and low high-density lipoprotein cholesterol (HDL-C) levels, apabetalone showed no greater increase in HDL-cholesterol (HDL-c) and apolipoprotein A-I (ApoA-I) levels or incremental regression of atherosclerosis than administration of placebo, while causing a statistically significant greater incidence of elevated liver enzymes. However, pooled analysis of the effect of apabetalone in three phase II clinical trials ASSERT, ASSURE, and SUSTAIN demonstrated increases in HDL-cholesterol (HDL-c) and apolipoprotein A-I (ApoA-I) levels, as well as decreases in the incidence of major adverse cardiac events (MACE). Reduction of MACE was more profound in patients with diabetes mellitus. In a short-term study in prediabetics, favorable changes in glucose metabolism were observed in patients receiving apabetalone. An international, multicenter phase III trial, “Effect of RVX000222 on Time to Major Adverse Cardiovascular Events in High-Risk Type 2 Diabetes Mellitus Subjects with Coronary Artery Disease” (BETonMACE) commenced in October 2015. The trial is designed to determine whether apabetalone in combination with statins can decrease cardiac events compared to treatment with statins alone.













