
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+
3, methanium CH+
5, acylium ions RCO+, and vinyl C
2H+
3 cations.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
In organic chemistry, a carbanion is an anion in which carbon is negatively charged.

Magic acid is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid and antimony pentafluoride. This conjugate Brønsted–Lewis superacid system was developed in the 1960s by Ronald Gillespie and his team at McMaster University, and has been used by George Olah to stabilise carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen.
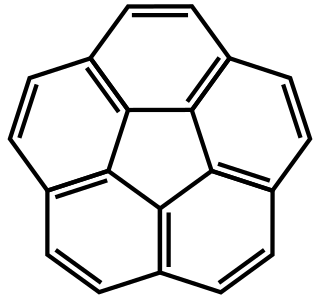
Corannulene is a polycyclic aromatic hydrocarbon with chemical formula C20H10. The molecule consists of a cyclopentane ring fused with 5 benzene rings, so another name for it is [5]circulene. It is of scientific interest because it is a geodesic polyarene and can be considered a fragment of buckminsterfullerene. Due to this connection and also its bowl shape, corannulene is also known as a buckybowl. Buckybowls are fragments of buckyballs. Corannulene exhibits a bowl-to-bowl inversion with an inversion barrier of 10.2 kcal/mol (42.7 kJ/mol) at −64 °C.
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon. They can be described as cationic [1,2]-sigmatropic rearrangements, proceeding suprafacially and with stereochemical retention. As such, a Wagner–Meerwein shift is a thermally allowed pericyclic process with the Woodward-Hoffmann symbol [ω0s + σ2s]. They are usually facile, and in many cases, they can take place at temperatures as low as –120 °C. The reaction is named after the Russian chemist Yegor Yegorovich Vagner; he had German origin and published in German journals as Georg Wagner; and Hans Meerwein.
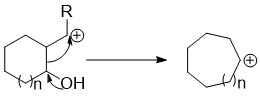
Ring expansion and ring contraction reactions expand or contract rings, usually in organic chemistry. The term usually refers to reactions involve making and breaking C-C bonds, Diverse mechanisms lead to these kinds of reactions.

A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a general term for diamagnetic carbon-based cations. In parallel with carbenium ions is another subset of carbocations, the carbonium ions with the formula R5+. In carbenium ions charge is localized. They are isoelectronic with monoboranes such as B(CH3)3.

In chemistry, a carbonium ion is a cation that has a pentacoordinated carbon atom. They are a type of carbocation. In older literature, the name "carbonium ion" was used for what is today called carbenium. Carbonium ions charge is delocalized in three-center, two-electron bonds. The more stable members are often bi- or polycyclic.

In organic chemistry, the term 2-norbornyl cation describes a carbonium ionic derivative of norbornane. A salt of the 2-norbornyl cation was crystallized and characterized by X-ray crystallography confirmed the non-classical structure.
Bullvalene is a hydrocarbon with the chemical formula C10H10. The molecule has a cage-like structure formed by the fusion of one cyclopropane and three cyclohepta-1,4-diene rings. Bullvalene is unusual as an organic molecule due to the C−C and C=C bonds forming and breaking rapidly on the NMR timescale; this property makes it a fluxional molecule.
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.
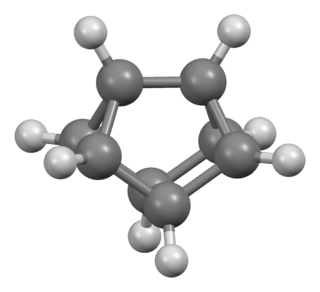
Cuneane is a saturated hydrocarbon with the formula C8H8 and a 3D structure resembling a wedge, hence the name. Cuneane may be produced from cubane by metal-ion-catalyzed σ-bond rearrangement. Similar reactions are known for homocubane and bishomocubane.

The vinyl cation is a carbocation with the positive charge on an alkene carbon. Its empirical formula of the parent ion is C
2H+
3. Vinyl cation are invoked as reactive intermediates in solvolysis of vinyl halides, as well as electrophilic addition to alkynes and allenes.
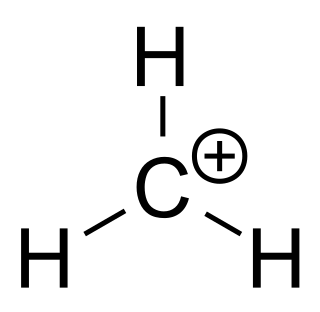
In organic chemistry, methenium is a cation with the formula CH+
3. It can be viewed as a methylene radical with an added proton, or as a methyl radical with one electron removed. It is a carbocation and an enium ion, making it the simplest of the carbenium ions.
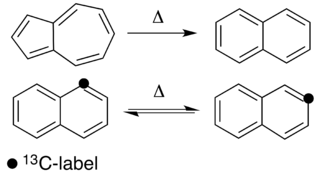
Thermal rearrangements of aromatic hydrocarbons are considered to be unimolecular reactions that directly involve the atoms of an aromatic ring structure and require no other reagent than heat. These reactions can be categorized in two major types: one that involves a complete and permanent skeletal reorganization (isomerization), and one in which the atoms are scrambled but no net change in the aromatic ring occurs (automerization). The general reaction schemes of the two types are illustrated in Figure 1.
David Markham Lemal is the Albert W. Smith Professor of Chemistry Emeritus and Research Professor of Chemistry at Dartmouth College. He received an A.B. degree (summa) from Amherst College in 1955 and a Ph.D. in chemistry from Harvard University in 1959. At Harvard he worked with R. B. Woodward on deoxy sugars and a synthesis of the alkaloid yohimbine.
Hydrogen-bridged cations are a type of charged species in which a hydrogen atom is simultaneously bonded to two atoms through partial sigma bonds. While best observable in the presence of superacids at room temperature, spectroscopic evidence has suggested that hydrogen-bridged cations exist in ordinary solvents. These ions have been the subject of debate as they constitute a type of charged species of uncertain electronic structure.
Bicycloaromaticity in chemistry is an extension of the concept of homoaromaticity with two aromatic ring currents situated in a non-planar molecule and sharing the same electrons. The concept originates with Melvin Goldstein who first reported about it in 1967. It is of some importance in academic research. Using MO theory the bicyclo[3.2.2]nonatrienyl cation was predicted to be destabilised and the corresponding anion predicted to be stabilised by bicycloaromaticity.
In chemistry, the decay technique is a method to generate chemical species such as radicals, carbocations, and other potentially unstable covalent structures by radioactive decay of other compounds. For example, decay of a tritium-labeled molecule yields an ionized helium atom, which might then break off to leave a cationic molecular fragment.










