Naphthalene is an organic compound with formula C
10H
8. It is the simplest polycyclic aromatic hydrocarbon, and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is best known as the main ingredient of traditional mothballs.

Hydroxylamine is an inorganic compound with the formula NH2OH. The material is a white crystalline, hygroscopic compound. Hydroxylamine is almost always provided and used as an aqueous solution. It is consumed almost exclusively to produce Nylon-6. It is also an intermediate in biological nitrification. The oxidation of NH3 to hydroxylamine is a step in biological nitrification.

A sulfonate is a salt or ester of a sulfonic acid. It contains the functional group R-SO−
3, where R is an organic group. Sulfonates are the conjugate base of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates.

Imidazole is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-adjacent nitrogen atoms in meta-substitution.

A sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
The formazans are compounds of the general formula [R-N=N-C(R')=N-NH-R"], formally derivatives of formazan [H2NN=CHN=NH], unknown in free form.

2-Naphthylamine is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, but it is a known carcinogen and has largely been replaced by less toxic compounds.
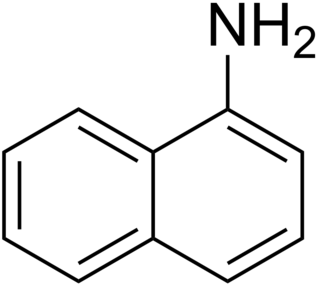
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer. It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.

2-Naphthol, or β-naphthol, is a fluorescent colorless (or occasionally yellow) crystalline solid with the formula C10H7OH. It is an isomer of 1-naphthol, differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, but more reactive. Both isomers are soluble in simple alcohols, ethers, and chloroform. 2-Naphthol is a widely used intermediate for the production of dyes and other compounds.

Benzenesulfonic acid (conjugate base benzenesulfonate) is an organosulfur compound with the formula C6H6O3S. It is the simplest aromatic sulfonic acid. It forms white deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in nonpolar solvents like diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic.

Naphthionic acid is an organic compound with the formula C10H6(SO3H)(NH2). It is one of several aminonaphthalenesulfonic acids, derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear gray. It is used in the synthesis of azo dyes such as Rocceline (a. k. a. Solid Red A), during which the amino group of the acid (in the form of a salt) is diazotated and then coupled with, in the case mentioned, β-naphthol. It is prepared by treating 1-aminonaphthalene with sulfuric acid.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.
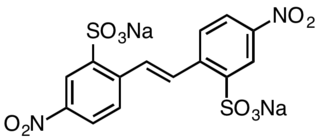
Disodium 4,4′-dinitrostilbene-2,2′-disulfonate is an organic compound with the formula (O2NC6H3(SO3Na)CH)2. This salt is a common precursor to a variety of textile dyes and optical brighteners
Aminonaphthalenesulfonic acids are compounds with the composition C10H6(NH2)(SO3H), being derived from naphthalene (C10H8) substituted by an amino and sulfonic acid groups. These compounds are colorless solids. They are useful precursors to dyes.

Tobias acid (2-amino-1-naphthalenesulfonic acid) is an organic compound with the formula C10H6(SO3H)(NH2). It is named after the German chemist Georg Tobias. It is one of several aminonaphthalenesulfonic acids, which are derivatives of naphthalene containing both amine and sulfonic acid functional groups. It is a white solid, although commercial samples can appear otherwise. It is used in the synthesis of azo dyes such as C.I. Acid Yellow 19 and C.I. Pigment Red 49. It is prepared via the Bucherer reaction of 2-hydroxynaphthalene-1-sulfonic acid with ammonia and ammonium sulfite.
In organic chemistry, peri-naphthalenes are particular derivatives of naphthalene with the formula C10H6-1,8-X2. Owing to the rigidity of the naphthalene skeleton, these substituents on the 1- and 8-positions are constrained to be relatively close 2.5 Å, which is within the van der Waals radius for many atoms. In contrast, ortho-substituents pendant from a benzene ring are separated by about 3.3 Å.
1,4-butane sultone is a six-membered δ-sultone and the cyclic ester of 4-hydroxybutanesulfonic acid. As a sulfo-alkylating agent, 1,4-butanesultone is used to introduce the sulfobutyl group (–(CH2)4–SO3−) into hydrophobic compounds possessing nucleophilic functional groups, for example hydroxy groups (as in the case of β-cyclodextrin) or amino groups (as in the case of polymethine dyes). In such, the sulfobutyl group is present as neutral sodium salt and considerably increases the water solubility of the derivatives.

Naphthalene-1-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the dihydrate C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being the more stable naphthalene-2-sulfonic acid. The compound is mainly used in the production of dyes.

Naphthalene-2-sulfonic acid is an organic compound with the formula C10H7SO3H. A colorless, water-soluble solid, it is often available as the mono- and trihydrates C10H7SO3H.2H2O. It is one of two monosulfonic acids of naphthalene, the other being naphthalene-1-sulfonic acid. The compound is mainly used in the production of dyes via nitration en route to aminonaphthalenesulfonic acids. The compound is prepared by sulfonation of naphthalene with sulfuric acid, however under equilibrating conditions that allow the 1-sulfonic acid isomer to convert to the more stable 2-sulfonic acid.