Related Research Articles

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. In electrochemical cells, electrodes are essential parts that can consist of a variety of materials (chemicals) depending on the type of cell. An electrode may be called either a cathode or anode according to the direction of the electric current, unrelated to the potential difference between electrodes.

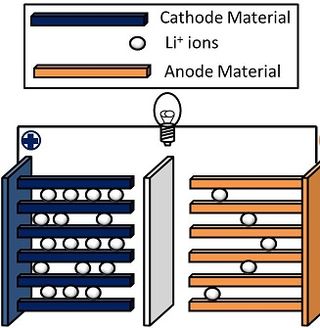
A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: over the following 30 years, their volumetric energy density increased threefold while their cost dropped tenfold. In late 2024 global demand passed 1 Terawatt-hour per year, while production capacity was more than twice that.

John Bannister Goodenough was an American materials scientist, a solid-state physicist, and a Nobel laureate in chemistry. From 1986 he was a professor of Materials Science, Electrical Engineering and Mechanical Engineering, at the University of Texas at Austin. He is credited with identifying the Goodenough–Kanamori rules of the sign of the magnetic superexchange in materials, with developing materials for computer random-access memory and with inventing cathode materials for lithium-ion batteries.
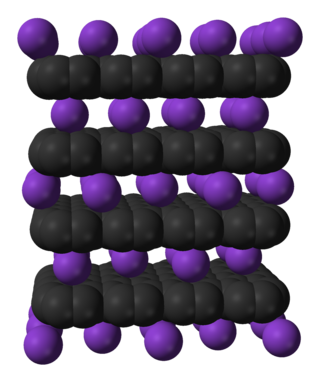
Intercalation is the reversible inclusion or insertion of a molecule into layered materials with layered structures. Examples are found in graphite and transition metal dichalcogenides.

Sir Michael Stanley Whittingham is a British-American chemist. He is a professor of chemistry and director of both the Institute for Materials Research and the Materials Science and Engineering program at Binghamton University, State University of New York. He also serves as director of the Northeastern Center for Chemical Energy Storage (NECCES) of the U.S. Department of Energy at Binghamton. He was awarded the Nobel Prize in Chemistry in 2019 alongside Akira Yoshino and John B. Goodenough.

The lithium iron phosphate battery or LFP battery is a type of lithium-ion battery using lithium iron phosphate as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. Because of their low cost, high safety, low toxicity, long cycle life and other factors, LFP batteries are finding a number of roles in vehicle use, utility-scale stationary applications, and backup power. LFP batteries are cobalt-free. As of September 2022, LFP type battery market share for EVs reached 31%, and of that, 68% were from EV makers Tesla and BYD alone. Chinese manufacturers currently hold a near monopoly of LFP battery type production. With patents having started to expire in 2022 and the increased demand for cheaper EV batteries, LFP type production is expected to rise further and surpass lithium nickel manganese cobalt oxides (NMC) type batteries in 2028.

Batteries provided the main source of electricity before the development of electric generators and electrical grids around the end of the 19th century. Successive improvements in battery technology facilitated major electrical advances, from early scientific studies to the rise of telegraphs and telephones, eventually leading to portable computers, mobile phones, electric cars, and many other electrical devices.

Lithium iron phosphate or lithium ferro-phosphate (LFP) is an inorganic compound with the formula LiFePO
4. It is a gray, red-grey, brown or black solid that is insoluble in water. The material has attracted attention as a component of lithium iron phosphate batteries, a type of Li-ion battery. This battery chemistry is targeted for use in power tools, electric vehicles, solar energy installations and more recently large grid-scale energy storage.

The lithium–sulfur battery is a type of rechargeable battery. It is notable for its high specific energy. The low atomic weight of lithium and moderate atomic weight of sulfur means that Li–S batteries are relatively light. They were used on the longest and highest-altitude unmanned solar-powered aeroplane flight by Zephyr 6 in August 2008.

Sodium-ion batteries (NIBs, SIBs, or Na-ion batteries) are several types of rechargeable batteries, which use sodium ions (Na+) as their charge carriers. In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the intercalating ion. Sodium belongs to the same group in the periodic table as lithium and thus has similar chemical properties. However, in some cases, such as aqueous batteries, SIBs can be quite different from LIBs.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and reducing cost.

NASICON is an acronym for sodium (Na) super ionic conductor, which usually refers to a family of solids with the chemical formula Na1+xZr2SixP3−xO12, 0 < x < 3. In a broader sense, it is also used for similar compounds where Na, Zr and/or Si are replaced by isovalent elements. NASICON compounds have high ionic conductivities, on the order of 10−3 S/cm, which rival those of liquid electrolytes. They are caused by hopping of Na ions among interstitial sites of the NASICON crystal lattice.
Magnesium batteries are batteries that utilize magnesium cations as charge carriers and possibly in the anode in electrochemical cells. Both non-rechargeable primary cell and rechargeable secondary cell chemistries have been investigated. Magnesium primary cell batteries have been commercialised and have found use as reserve and general use batteries.
The glass battery is a type of solid-state battery. It uses a glass electrolyte and lithium or sodium metal electrodes.

Inverse vulcanization is a process that produces polysulfide polymers, which also contain some organic linkers. In contrast, sulfur vulcanization produces material that is predominantly organic but has a small percentage of polysulfide crosslinks.
Linda Faye Nazar is a Senior Canada Research Chair in Solid State Materials and Distinguished Research Professor of Chemistry at the University of Waterloo. She develops materials for electrochemical energy storage and conversion. Nazar demonstrated that interwoven composites could be used to improve the energy density of lithium–sulphur batteries. She was awarded the 2019 Chemical Institute of Canada Medal.
The lithium nickel cobalt aluminium oxides (abbreviated as Li-NCA, LNCA, or NCA) are a group of mixed metal oxides. Some of them are important due to their application in lithium-ion batteries. NCAs are used as active material in the positive electrode (which is the cathode when the battery is discharged). NCAs are composed of the cations of the chemical elements lithium, nickel, cobalt and aluminium. The compounds of this class have a general formula LiNixCoyAlzO2 with x + y + z = 1. In case of the NCA comprising batteries currently available on the market, which are also used in electric cars and electric appliances, x ≈ 0.84, and the voltage of those batteries is between 3.6 V and 4.0 V, at a nominal voltage of 3.6 V or 3.7 V. A version of the oxides currently in use in 2019 is LiNi0.84Co0.12Al0.04O2.
Lithium nickel manganese cobalt oxides (abbreviated NMC, Li-NMC, LNMC, or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt with the general formula LiNixMnyCo1-x-yO2. These materials are commonly used in lithium-ion batteries for mobile devices and electric vehicles, acting as the positively charged cathode.

This is a history of the lithium-ion battery.
References
- 1 2 "Professor Arumugam Manthiram Delivered the Nobel Prize Lecture". Dinamalar . Archived from the original on May 24, 2022.
- ↑ "Arumugam Manthiram Elected as a Fellow of AAAS". Texas Materials Institute .
- 1 2 "Manthiram Presents Goodenough's Nobel Lecture". Electrochemical Society . 13 December 2019.
- ↑ "Three Indian American Professors Named 2016 Materials Research Society Fellows". India West .
- ↑ "Arumugam Manthiram". Chemistry Tree .
- ↑ Arumugam Manthiram, Challenges and Opportunities of Electrical Energy Storage Technologies) on YouTube
- ↑ "John B. Goodenough Nobel Lecture". Nobel Prize .
- ↑ Manthiram, A.; Goodenough, J. B. (1989). "Lithium insertion into Fe2(SO4)3 frameworks". Journal of Power Sources. 26 (3–4): 403–408. Bibcode:1989JPS....26..403M. doi:10.1016/0378-7753(89)80153-3.
- ↑ Manthiram, A.; Goodenough, J. B. (1987). "Lithium insertion into Fe2(MO4)3 frameworks: Comparison of M = W with M = Mo". Journal of Solid State Chemistry. 71 (2): 349–360. Bibcode:1987JSSCh..71..349M. doi: 10.1016/0022-4596(87)90242-8 .
- ↑ Masquelier, Christian; Croguennec, Laurence (2013). "Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries". Chemical Reviews. 113 (8): 6552–6591. doi:10.1021/cr3001862. PMID 23742145.
- ↑ Chebiam, R. V.; Kannan, A. M.; Prado, F.; Manthiram, A. (2001). "Comparison of the chemical stability of the high energy density cathodes of lithium-ion batteries". Electrochemistry Communications. 3 (11): 624–627. doi:10.1016/S1388-2481(01)00232-6.
- ↑ Chebiam, R. V.; Prado, F.; Manthiram, A. (2001). "Soft Chemistry Synthesis and Characterization of Layered Li1−xNi1−yCoyO2−δ (0 ≤ x ≤ 1 and 0 ≤ y ≤ 1)". Chemistry of Materials. 13: 2951–2957. doi:10.1021/cm0102537.
- ↑ Manthiram, Arumugam (2020). "A reflection on lithium-ion battery cathode chemistry". Nature Communications. 11 (1): 1550. Bibcode:2020NatCo..11.1550M. doi: 10.1038/s41467-020-15355-0 . PMC 7096394 . PMID 32214093.
- 1 2 Bhargav, Amruth; Jiarui, He (2020). "Lithium-Sulfur Batteries: Attaining the Critical Metrics". Joule. 4 (2): 285–291. Bibcode:2020Joule...4..285B. doi: 10.1016/j.joule.2020.01.001 .
- ↑ Manthiram, Arumugam; Fu, Yongzhu; Chung, Sheng-Heng; Zu, Chenxi; Su, Yu-Sheng (2014). "Rechargeable Lithium–Sulfur Batteries". Chemical Reviews. 114 (23): 11751–11787. doi:10.1021/cr500062v. PMID 25026475.
- ↑ Su, Yu-Sheng; Manthiram, Arumugam (2012). "Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer". Nature Communications. 3: 1166. Bibcode:2012NatCo...3.1166S. doi: 10.1038/ncomms2163 . PMID 23132016.
- ↑ Zhou, Guangmin; Paek, Eunsu; Hwang, Gyeong; Manthiram, Arumugam (2015). "Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur codoped graphene sponge". Nature Communications. 6: 7760. Bibcode:2015NatCo...6.7760Z. doi: 10.1038/ncomms8760 . PMC 4518288 . PMID 26182892.