Multiple hypotheses explain the possible connections between sleep and learning in humans. Research indicates that sleep does more than allow the brain to rest; it may also aid the consolidation of long-term memories.
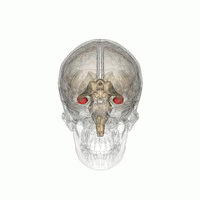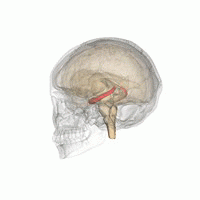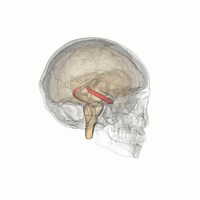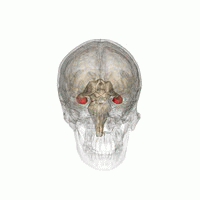
The hippocampus is a major component of the brain of humans and other vertebrates. Humans and other mammals have two hippocampi, one in each side of the brain. The hippocampus is part of the limbic system, and plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus is located in the allocortex, with neural projections into the neocortex, in humans as well as other primates. The hippocampus, as the medial pallium, is a structure found in all vertebrates. In humans, it contains two main interlocking parts: the hippocampus proper, and the dentate gyrus.

Orexin, also known as hypocretin, is a neuropeptide that regulates arousal, wakefulness, and appetite. The most common form of narcolepsy, type 1, in which the individual experiences brief losses of muscle tone, is caused by a lack of orexin in the brain due to destruction of the cells that produce it. It exists in the forms of orexin-A and orexin-B.
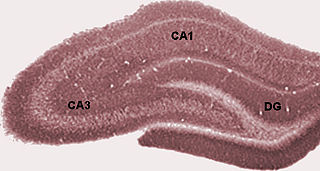
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories, the spontaneous exploration of novel environments and other functions.

The nucleus accumbens is a region in the basal forebrain rostral to the preoptic area of the hypothalamus. The nucleus accumbens and the olfactory tubercle collectively form the ventral striatum. The ventral striatum and dorsal striatum collectively form the striatum, which is the main component of the basal ganglia. The dopaminergic neurons of the mesolimbic pathway project onto the GABAergic medium spiny neurons of the nucleus accumbens and olfactory tubercle. Each cerebral hemisphere has its own nucleus accumbens, which can be divided into two structures: the nucleus accumbens core and the nucleus accumbens shell. These substructures have different morphology and functions.

Adult neurogenesis is the process in which neurons are generated from neural stem cells in the adult. This process differs from prenatal neurogenesis.

Part of the human brain, the basal forebrain structures are located in the forebrain to the front of and below the striatum. They include the ventral basal ganglia, nucleus basalis, diagonal band of Broca, substantia innominata, and the medial septal nucleus. These structures are important in the production of acetylcholine, which is then distributed widely throughout the brain. The basal forebrain is considered to be the major cholinergic output of the central nervous system (CNS) centred on the output of the nucleus basalis. The presence of non-cholinergic neurons projecting to the cortex have been found to act with the cholinergic neurons to dynamically modulate activity in the cortex.

The neuroimmune system is a system of structures and processes involving the biochemical and electrophysiological interactions between the nervous system and immune system which protect neurons from pathogens. It serves to protect neurons against disease by maintaining selectively permeable barriers, mediating neuroinflammation and wound healing in damaged neurons, and mobilizing host defenses against pathogens.

Gero Andreas Miesenböck is an Austrian scientist. He is currently Waynflete Professor of Physiology and Director of the Centre for Neural Circuits and Behaviour (CNCB) at the University of Oxford and a fellow of Magdalen College, Oxford.
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient with Retinitis pigmentosa.
Protective autoimmunity is a condition in which cells of the adaptive immune system contribute to maintenance of the functional integrity of a tissue, or facilitate its repair following an insult. The term ‘protective autoimmunity’ was coined by Prof. Michal Schwartz of the Weizmann Institute of Science (Israel), whose pioneering studies were the first to demonstrate that autoimmune T lymphocytes can have a beneficial role in repair, following an injury to the central nervous system (CNS). Most of the studies on the phenomenon of protective autoimmunity were conducted in experimental settings of various CNS pathologies and thus reside within the scientific discipline of neuroimmunology.
The inflammatory reflex is a neural circuit that regulates the immune response to injury and invasion. All reflexes have an afferent and efferent arc. The Inflammatory reflex has a sensory afferent arc, which is activated by cytokines, and a motor or efferent arc, which transmits action potentials in the vagus nerve to suppress cytokine production. Increased signaling in the efferent arc inhibits inflammation and prevents organ damage.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
Sharp waves and ripples (SWRs) are oscillatory patterns produced by extremely synchronised activity of neurons in the mammalian hippocampus and neighbouring regions which occur spontaneously in idle waking states or during NREM sleep. They can be observed with a variety of imaging methods, such as EEG. They are composed of large amplitude sharp waves in local field potential and produced by tens of thousands of neurons firing together within 30–100 ms window. They are some of the most synchronous oscillations patterns in the brain, making them susceptible to pathological patterns such as epilepsy.They have been extensively characterised and described by György Buzsáki and have been shown to be involved in memory consolidation in NREM sleep and the replay of memories acquired during wakefulness.

Feng Zhang is a Chinese–American biochemist. Zhang currently holds the James and Patricia Poitras Professorship in Neuroscience at the McGovern Institute for Brain Research and in the departments of Brain and Cognitive Sciences and Biological Engineering at the Massachusetts Institute of Technology. He also has appointments with the Broad Institute of MIT and Harvard. He is most well known for his central role in the development of optogenetics and CRISPR technologies.

Raz Yirmiya is an Israeli behavioral neuroscientist and director of the Laboratory for Psychoneuroimmunology at the Hebrew University of Jerusalem in Israel. He is best known for providing the first experimental evidence for the role of immune system activation in depression, for discovering that disturbances in brain microglia cells underlie some forms of depression, and for elucidating the involvement of inflammatory cytokines in regulation of cognitive and emotional processes.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). In short, it is brain growth in relation to its organization. This occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.

Michal Schwartz is a professor of neuroimmunology at the Weizmann Institute of Science. She is active in the field of neurodegenerative diseases, particularly utilizing the immune system to help the brain fight terminal neurodegenerative brain diseases, such as Alzheimer's disease and dementia.
Christine Denny is an American neuroscientist and associate professor of Clinical Neurobiology in Psychiatry in the Department of Psychiatry at Columbia University Irving Medical Center in New York City. Denny investigates the molecular mechanisms underlying learning and memory. She developed a novel technique to label neurons that encode specific memories. She used this technique to probe what happens to hippocampal memory traces in different disease states.

Erin M. Gibson is a glial and circadian biologist as well as an assistant professor in the Department of Psychiatry and Behavioral Sciences and the Stanford Center for Sleep Sciences and Medicine at Stanford University. Gibson investigates the role of glial cells in sculpting neural circuits and mechanistically probes how the circadian rhythm modulates glial biology.













