
The stromal cell-derived factor 1 (SDF-1), also known as C-X-C motif chemokine 12 (CXCL12), is a chemokine protein that in humans is encoded by the CXCL12 gene on chromosome 10. It is ubiquitously expressed in many tissues and cell types. Stromal cell-derived factors 1-alpha and 1-beta are small cytokines that belong to the chemokine family, members of which activate leukocytes and are often induced by proinflammatory stimuli such as lipopolysaccharide, TNF, or IL1. The chemokines are characterized by the presence of 4 conserved cysteines that form 2 disulfide bonds. They can be classified into 2 subfamilies. In the CC subfamily, the cysteine residues are adjacent to each other. In the CXC subfamily, they are separated by an intervening amino acid. The SDF1 proteins belong to the latter group. CXCL12 signaling has been observed in several cancers. The CXCL12 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

Microglia are a type of neuroglia located throughout the brain and spinal cord. Microglia account for about 10-15% of cells found within the brain. As the resident macrophage cells, they act as the first and main form of active immune defense in the central nervous system (CNS). Microglia originate in the yolk sac under a tightly regulated molecular process. These cells are distributed in large non-overlapping regions throughout the CNS. Microglia are key cells in overall brain maintenance—they are constantly scavenging the CNS for plaques, damaged or unnecessary neurons and synapses, and infectious agents. Since these processes must be efficient to prevent potentially fatal damage, microglia are extremely sensitive to even small pathological changes in the CNS. This sensitivity is achieved in part by the presence of unique potassium channels that respond to even small changes in extracellular potassium. Recent evidence shows that microglia are also key players in the sustainment of normal brain functions under healthy conditions. Microglia also constantly monitor neuronal functions through direct somatic contacts and exert neuroprotective effects when needed.
Neuroimmunology is a field combining neuroscience, the study of the nervous system, and immunology, the study of the immune system. Neuroimmunologists seek to better understand the interactions of these two complex systems during development, homeostasis, and response to injuries. A long-term goal of this rapidly developing research area is to further develop our understanding of the pathology of certain neurological diseases, some of which have no clear etiology. In doing so, neuroimmunology contributes to development of new pharmacological treatments for several neurological conditions. Many types of interactions involve both the nervous and immune systems including the physiological functioning of the two systems in health and disease, malfunction of either and or both systems that leads to disorders, and the physical, chemical, and environmental stressors that affect the two systems on a daily basis.

The neuroimmune system is a system of structures and processes involving the biochemical and electrophysiological interactions between the nervous system and immune system which protect neurons from pathogens. It serves to protect neurons against disease by maintaining selectively permeable barriers, mediating neuroinflammation and wound healing in damaged neurons, and mobilizing host defenses against pathogens.
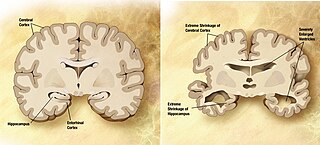
A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, tauopathies, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.

Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective, to induce a specific cell signal, to cause the immune system to attack specific cells, or to bring a drug to a specific cell type.

Toll-like receptor 4 (TLR4), also designated as CD284, is a key activator of the innate immune response and plays a central role in the fight against bacterial infections. TLR4 is a transmembrane protein of approximately 95 kDa that is encoded by the TLR4 gene.
Certain sites of the mammalian body have immune privilege, meaning they are able to tolerate the introduction of antigens without eliciting an inflammatory immune response. Tissue grafts are normally recognised as foreign antigens by the body and attacked by the immune system. However, in immune privileged sites, tissue grafts can survive for extended periods of time without rejection occurring. Immunologically privileged sites include:

Programmed death-ligand 1 (PD-L1) also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1) is a protein that in humans is encoded by the CD274 gene.
Colony stimulating factor 1 receptor (CSF1R), also known as macrophage colony-stimulating factor receptor (M-CSFR), and CD115, is a cell-surface protein encoded by the human CSF1R gene. CSF1R is a receptor that can be activated by two ligands: colony stimulating factor 1 (CSF-1) and interleukin-34 (IL-34). CSF1R is highly expressed in myeloid cells, and CSF1R signaling is necessary for the survival, proliferation, and differentiation of many myeloid cell types in vivo and in vitro. CSF1R signaling is involved in many diseases and is targeted in therapies for cancer, neurodegeneration, and inflammatory bone diseases.

Programmed cell death protein 1(PD-1),. PD-1 is a protein encoded in humans by the PDCD1 gene. PD-1 is a cell surface receptor on T cells and B cells that has a role in regulating the immune system's response to the cells of the human body by down-regulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. This prevents autoimmune diseases, but it can also prevent the immune system from killing cancer cells.

Triggering receptor expressed on myeloid cells 2(TREM2) is a protein that in humans is encoded by the TREM2 gene. TREM2 is expressed on macrophages, immature monocyte-derived dendritic cells, osteoclasts, and microglia, which are immune cells in the central nervous system. In the liver, TREM2 is expressed by several cell types, including macrophages, that respond to injury. In the intestine, TREM2 is expressed by myeloid-derived dendritic cells and macrophage. TREM2 is overexpressed in many tumor types and has anti-inflammatory activities. It might therefore be a good therapeutic target.
Protective autoimmunity is a condition in which cells of the adaptive immune system contribute to maintenance of the functional integrity of a tissue, or facilitate its repair following an insult. The term ‘protective autoimmunity’ was coined by Prof. Michal Schwartz of the Weizmann Institute of Science (Israel), whose pioneering studies were the first to demonstrate that autoimmune T lymphocytes can have a beneficial role in repair, following an injury to the central nervous system (CNS). Most of the studies on the phenomenon of protective autoimmunity were conducted in experimental settings of various CNS pathologies and thus reside within the scientific discipline of neuroimmunology.
Neuroinflammation is inflammation of the nervous tissue. It may be initiated in response to a variety of cues, including infection, traumatic brain injury, toxic metabolites, or autoimmunity. In the central nervous system (CNS), including the brain and spinal cord, microglia are the resident innate immune cells that are activated in response to these cues. The CNS is typically an immunologically privileged site because peripheral immune cells are generally blocked by the blood–brain barrier (BBB), a specialized structure composed of astrocytes and endothelial cells. However, circulating peripheral immune cells may surpass a compromised BBB and encounter neurons and glial cells expressing major histocompatibility complex molecules, perpetuating the immune response. Although the response is initiated to protect the central nervous system from the infectious agent, the effect may be toxic and widespread inflammation as well as further migration of leukocytes through the blood–brain barrier may occur.

The meningeal lymphatic vessels are a network of conventional lymphatic vessels located parallel to the dural venous sinuses and middle meningeal arteries of the mammalian central nervous system (CNS). As a part of the lymphatic system, the meningeal lymphatics are responsible for draining immune cells, small molecules, and excess fluid from the CNS into the deep cervical lymph nodes. Cerebrospinal fluid, and interstitial fluid are exchanged, and drained by the meningeal lymphatic vessels.

The pathophysiology of Parkinson's disease is death of dopaminergic neurons as a result of changes in biological activity in the brain with respect to Parkinson's disease (PD). There are several proposed mechanisms for neuronal death in PD; however, not all of them are well understood. Five proposed major mechanisms for neuronal death in Parkinson's Disease include protein aggregation in Lewy bodies, disruption of autophagy, changes in cell metabolism or mitochondrial function, neuroinflammation, and blood–brain barrier (BBB) breakdown resulting in vascular leakiness.

Jonathan Kipnis is a neuroscientist, immunologist, and professor of pathology and immunology at the Washington University School of Medicine. His lab studies interactions between the immune system and nervous system. He is best known for his lab's discovery of meningeal lymphatic vessels in humans and mice, which has impacted research on neurodegenerative diseases such as Alzheimer's disease and multiple sclerosis, neuropsychiatric disorders, such as anxiety, and neurodevelopmental disorders such as autism and Rett syndrome.
Microglia are the primary immune cells of the central nervous system, similar to peripheral macrophages. They respond to pathogens and injury by changing morphology and migrating to the site of infection/injury, where they destroy pathogens and remove damaged cells.
Melanie Greter is a Swiss neuroimmunologist and a Swiss National Science Foundation Professor in the Institute of Experimental Immunology at the University of Zurich. Greter explores the ontogeny and function of microglia and border-associated macrophages of the central nervous system to understand how they maintain homeostasis and contribute to brain-related diseases.
Malú G. Tansey is an American Physiologist and Neuroscientist as well as the Director of the Center for Translational Research in Neurodegenerative Disease at the University of Florida. Tansey holds the titles of Evelyn F. and William L. McKnight Brain Investigator and Norman Fixel Institute for Neurological Diseases Investigator. As the principal investigator of the Tansey Lab, Tansey guides a research program centered around investigating the role of neuroimmune interactions in the development and progression of neurodegenerative and neuropsychiatric disease. Tansey's work is primarily focused on exploring the cellular and molecular basis of peripheral and central inflammation in the pathology of age-related neurodegenerative diseases like Alzheimer's disease and amyotrophic lateral sclerosis.













