
Elias James Corey is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.

Epibatidine is a chlorinated alkaloid that is secreted by the Ecuadoran frog Epipedobates anthonyi and poison dart frogs from the Ameerega genus. It was discovered by John W. Daly in 1974, but its structure was not fully elucidated until 1992. Whether epibatidine is the first observed example of a chlorinated alkaloid remains controversial, due to challenges in conclusively identifying the compound from the limited samples collected by Daly. By the time that high-resolution spectrometry was used in 1991, there remained less than one milligram of extract from Daly's samples, raising concerns about possible contamination. Samples from other batches of the same species of frog failed to yield epibatidine.

The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone. The reaction was discovered by Ihsan Ullah Khand (1935-1980), who was working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. The seminal report dates back to 1970, however a detailed follow up was reported in 1973. Initial findings by Pauson and Khand were intermolecular in nature, however many intramolecular examples have been highlighted in both synthesis and methodology reports, starting a decade later from reaction discovery. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient and catalytic utilizing different chiral auxiliaries for stereo induction, main group transition-metals, and additives to enhance rate of reactivity and yield. For a more extensive review on PKR, refer to Torres' book.

Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine (-NR-) and two methylene bridges. The parent compound is aziridine, with molecular formula C
2H
4NH. Several drugs feature aziridine rings, including mitomycin C, porfiromycin, and azinomycin B (carzinophilin).
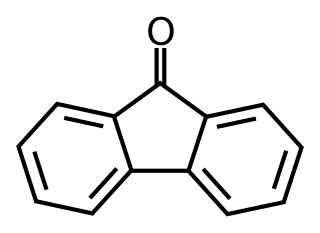
Fluorenone is an aromatic organic compound with the chemical formula C13H8O. It is used to make antimalaria drugs. It can be synthesised from fluorene with the addition of glacial acetic acid and sodium hypochlorite solution, undergoing an oxidation reaction. An alternate synthesis is described in scheme 43 of the Linopirdine patent.
The reduction of nitro compounds are chemical reactions of wide interest in organic chemistry. The conversion can be effected by many reagents. The nitro group was one of the first functional groups to be reduced. Alkyl and aryl nitro compounds behave differently. Most useful is the reduction of aryl nitro compounds.

Mesembrine is an alkaloid present in Sceletium tortuosum (kanna). It has been shown to act as a serotonin reuptake inhibitor (Ki = 1.4 nM), and more recently, has also been found to behave as a weak inhibitor of the enzyme phosphodiesterase 4 (PDE4) (Ki = 7,800 nM). Still more recently, in an in vitro study published in 2015, scientist concluded that "a high-mesembrine Sceletium extract exerts its anti-depressant effect by acting primarily as monoamine releasing agent, rather than as selective serotonin-reuptake inhibitor." As such, mesembrine likely plays a dominant role in the antidepressant effects of kanna. The levorotatory isomer, (−)-mesembrine, is the natural form.
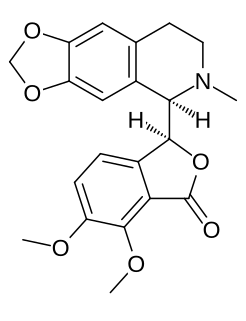
Hydrastine is an isoquinoline alkaloid which was discovered in 1851 by Alfred P. Durand. Hydrolysis of hydrastine yields hydrastinine, which was patented by Bayer as a haemostatic drug during the 1910s. It is present in Hydrastis canadensis and other plants of the family Ranunculaceae.
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula CH3SO2Cl. Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group CH3SO2, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene (methylenedioxosulfur(VI)).
In enzymology, a reticuline oxidase (EC 1.21.3.3) is an enzyme that catalyzes the chemical reaction

Alvocidib is a flavonoid alkaloid CDK9 kinase inhibitor under clinical development by Tolero Pharmaceuticals for the treatment of acute myeloid leukemia. It has been studied also for the treatment of arthritis and atherosclerotic plaque formation. The target of alvocidib is the positive transcription elongation factor P-TEFb. Treatment of cells with alvocidib leads to inhibition of P-TEFb and the loss of mRNA production.
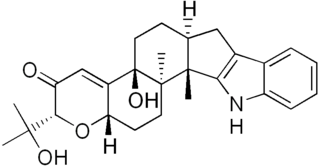
Paxilline is a toxic, tremorgenic diterpene indole polycyclic alkaloid molecule produced by Penicillium paxilli which was first characterized in 1975. Paxilline is one of a class of tremorigenic mycotoxins, is a potassium channel blocker, and is potentially genotoxic.

Woollins' reagent is an organic compound containing phosphorus and selenium. Analogous to Lawesson's reagent, it is used mainly as a selenation reagent. It is named after Professor John Derek Woollins.
The imine Diels–Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.
Fétizon oxidation is the oxidation of primary and secondary alcohols utilizing the compound silver(I) carbonate absorbed onto the surface of celite also known as Fétizon's reagent first employed by Marcel Fétizon in 1968. It is a mild reagent, suitable for both acid and base sensitive compounds. Its great reactivity with lactols makes the Fétizon oxidation a useful method to obtain lactones from a diol. The reaction is inhibited significantly by polar groups within the reaction system as well as steric hindrance of the α-hydrogen of the alcohol.
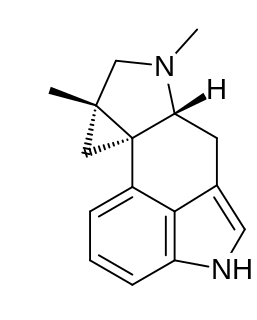
Cycloclavine is an ergot alkaloid. It was first isolated in 1969 from seeds of Ipomoea hildebrandtii vatke. The first total synthesis of (±)-cycloclavine was published in 2008 by Szántay. Further reports came from Wipf and Petronijevic, Cao and Brewer. In 2016, Wipf and McCabe completed an 8-step asymmetric synthesis of (–)-cycloclavine, and in 2018, they expanded this approach toward (+)-cycloclavine and a biological characterization of the binding profile of both enantiomers on 16 brain receptors. Natural (+)- and unnatural (–)-cycloclavine demonstrated significant stereospecificity and unique binding profiles in comparison to LSD, psilocin, and DMT. Differential 5-HT receptor affinities, as well as novel sigma-1 receptor properties, suggest potential future therapeutic opportunities of clavine alkaloid scaffolds.
Penicillium viridicatum is a psychrophilic species of fungus in the genus, penicillic acid and citrinin. Penicillium viridicatum can spoil grapes and melons.
Aspergillus leporis is an anamorph species of fungus in the genus Aspergillus. It is from the Flavi section. The species was first described in 1979. It has been isolated from the dung of Lepus townsendii. Aspergillus leporis produces leporin A and leporin B. It has also been reported to produce antibiotic Y, kojic acid, and pseurotin.
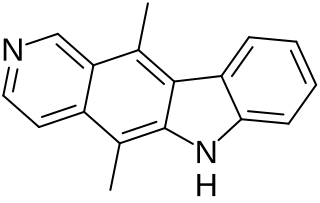
Ellipticine is an alkaloid first extracted from trees of the species Ochrosia elliptica and Rauvolfia sandwicensis, which inhibits the enzyme topoisomerase II via intercalative binding to DNA.

16-Methylene-17α-hydroxyprogesterone acetate is a progestin of the 17α-hydroxyprogesterone group which was never marketed. Given orally, it shows about 2.5-fold the progestogenic activity of parenteral progesterone in animal bioassays. It is a parent compound of the following clinically used progestins:













