
Tapinella atrotomentosa, commonly known as the velvet roll-rim or velvet-footed tap, is a species of fungus in the family Tapinellaceae. Although it has gills, it is a member of the pored mushroom order Boletales. August Batsch described the species in 1783. It has been recorded from Asia, Central America, Europe and North America. Tough and inedible, it grows on tree stumps of conifers. The mushroom contains several compounds that act as deterrents of feeding by insects.

Serpula lacrymans is a species of fungi known for causing dry rot. It is a basidiomycete in the order Boletales. It has the ability to rapidly colonise sites through unique and highly specialised mycelium which also leads to greater degradation rates of wood cellulose.

Suillus grevillei, commonly known as Greville's bolete, tamarack jack, or larch bolete, is a mycorrhizal mushroom with a tight, brilliantly coloured cap, shiny and wet looking with its mucous slime layer. The hymenium easily separates from the flesh of the cap, with a central stalk that is quite slender. The species has a ring or a tight-fitting annular zone.

The Dacrymycetes are a class of fungi in the Basidiomycota. The class currently contains the single order Dacrymycetales, with a second proposed order Unilacrymales now treated at the family level. The order contains four families and has a cosmopolitan distribution.
Chemical tests in mushroom identification are methods that aid in determining the variety of some fungi. The most useful tests are Melzer's reagent and potassium hydroxide.

Pulvinone, an organic compound belonging to the esters, lactones, alcohols and butenolides classes, is a yellow crystalline solid. Although the pulvinone is not a natural product, several naturally occurring hydroxylated derivatives are known. These hydroxylated pulvinones are produced by fungal species, such as the in Europe common Larch Bolete, or by moulds such as Aspergillus terreus.

Thelephoric acid is a terphenylquinone pigment that is found in several fungi, such as Omphalotus subilludens and Polyozellus multiplex. Thelephoric acid has been shown to inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. It is derived from atromentin, and its precursor can be from cyclovariegatin. Fragmentation patterns have suggested that polymers of thelephoric acid exists.

Gyroporus cyanescens, commonly known as the bluing bolete or the cornflower bolete, is a species of bolete fungus in the family Gyroporaceae. First described from France in 1788, the species is found in Asia, Australia, Europe, and eastern North America, where it grows on the ground in coniferous and mixed forests.
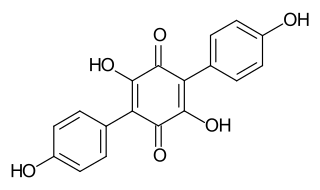
Atromentin is a natural chemical compound found in Agaricomycetes fungi in the orders Agaricales and Thelephorales. It can also be prepared by laboratory synthesis. Chemically, it is a polyphenol and a benzoquinone.

Omphalotus subilludens, commonly known as the Southern Jack O'lantern mushroom, is a basidiomycete fungi in the genus Omphalotus. It has been definitively recorded in Florida and Texas with reports of species in Arizona and Mexico. It fruits on dead and dying trees during warmer parts of the year, producing a fairly large orange to brown-orange fruiting body that occurs in clusters. It is most closely related to O. olivascans, O. olearius, and O. japonicus and has high cross compatibility with O. olivescans and O. olearis. It is poisonous to humans and animals when eaten but rarely produces life-threatening symptoms, usually poisonings are resolved in 24-48 hours, with the majority of symptoms being gastrointestinal. Compounds in these mushrooms have pharmacological potential with potential applications in anti-coagulants, cancer therapies, and antibiotics. It is also bioluminescent producing a faint glow around the gills through the oxidation of luciferase.
Boletocrocin is any one of a group of seven closely related organic compounds, individually named boletocrocin A through boletrocrocin G. These compounds are polyene dicarboxylic acids that include both lipophilic and polar amino acids. They were extracted from the brightly colored mushrooms Boletus laetissimus and B. rufoaureus. The boletocrocins' conjugated systems account for the intense color.
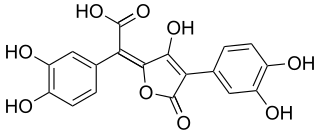
Variegatic acid is an orange pigment found in some mushrooms. It is responsible for the bluing reaction seen in many bolete mushrooms when they are injured. When mushroom tissue containing variegatic acid is exposed to air, the chemical is enzymatically oxidized to blue quinone methide anions, specifically chinonmethid anions. It is derived from xerocomic acid, which is preceded by atromentic acid and atromentin, and its genetic basis is unknown. In its oxidized form is variegatorubin, similar to xerocomorubin.

Pulvinic acids are natural chemical pigments found in some lichens, derived biosynthetically from the aromatic amino acids phenylalanine and tyrosine, via dimerization and oxidative ring-cleavage of arylpyruvic acids, a process that also produces the related pulvinones.

The conservation and restoration of frescoes is the process of caring for and maintaining frescos, and includes documentation, examination, research, and treatment to insure their long-term viability, when desired.
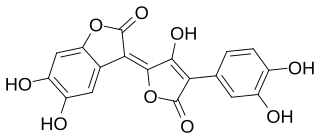
Variegatorubin is a pulvinic acid derivative. It is a red pigment that is present in many members of the Boletales, an order of the division Basidiomycota. It is generated from the oxidation of variegatic acid. Bolete species that contain variegatorubin include Neoboletus luridiformis, Chalciporus piperatus, Rhizopogon roseolus, Exsudoporus frostii, Suillellus luridus, Rubroboletus rhodoxanthus, and R. satanas. Variegatorubin was discovered by Wolfgang Steglich and colleagues, and described as a new compound in 1970.
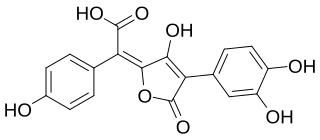
Xerocomic acid is a red-orange pigment found in fungi of the order Boletales. It is the precursor to variegatic acid, and is preceded by atromentic acid and atromentin. As an example, it is isolated from Serpula lacrymans. It is soluble in methanol. An oxidase acting on xerocomic acid is responsible for the "bluing" reaction seen in mushrooms.

Xerocomorubin is a pigment from the fungus order Boletales. It is the oxidized form of isoxerocomic acid. Air oxidation is responsible its formation, and it oxidizes faster to a similar pulvinic acid type pigment oxidized variant, variegatorubin. The long wavelength has an absorption at 497 nm, 106 nm higher than its precursor isoxerocomic acid. Synthesis experiments have shown tetra-acetylation by acetic anhydride and sulfuric acid. Although xerocomorubin and variegatorubin give off the same deep red color and could simultaneously occur in a mushroom, extracts from the deep red colored mushroom Boletus rubellus Krombh. identified only variegatorubin by thin layer chromatography (TLC), leading to the question the natural abundance of xerocomorubin.
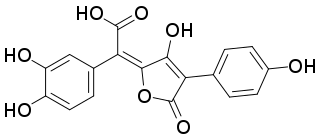
Isoxerocomic acid is a red-orange pigment found in Boletales. It is the precursor to variegatic acid, and is preceded by atromentic acid and atromentin. As an example, it is isolated from Serpula lacrymans. It is soluble in methanol. It is the isomer of xerocomic acid and precursor to xerocomorubin.
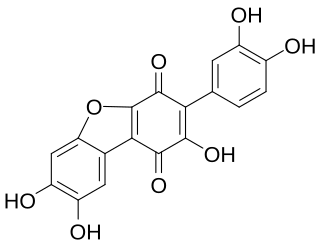
Cyclovariegatin is a pigment. Its chemical name is 1,4-dihydro-2,7,8-trihydroxy-3-(3,4-dihydroxyphenyl)-l,4-dioxodibenzofuran. It is distinguishable by its UV-Vis spectra with maxima at 257, 296, and 430 nm. The variants cyclovariegatin-pentaacetate, cyclovariegatin-2,3',8-triacetate, and cyclovariegatin-2-acetate have also been described. It is derived from atromentin. It has been isolated from the browned skin of Suillus grevillei var. badius, and becomes the pigment thelephoric acid.

















