
Scleroderma citrinum, commonly known as the common earthball, pigskin poison puffball, or common earth ball, is the most common species of earthball fungus in the UK and occurs widely in woods, heathland and in short grass from autumn to winter. Scleroderma citrinum has two synonyms, Scleroderma aurantium (Vaill.) and Scleroderma vulgare Horn.

Caloboletus calopus, commonly known as the bitter bolete, bitter beech bolete or scarlet-stemmed bolete, is a fungus of the bolete family, found in Asia, Northern Europe and North America. Appearing in coniferous and deciduous woodland in summer and autumn, the stout fruit bodies are attractively coloured, with a beige to olive cap up to 15 cm (6 in) across, yellow pores, and a reddish stipe up to 15 cm (6 in) long and 5 cm (2 in) wide. The pale yellow flesh stains blue when broken or bruised.

Chalciporus piperatus, commonly known as the peppery bolete, is a small pored mushroom of the family Boletaceae found in mixed woodland in Europe and North America. It has been recorded under introduced trees in Brazil, and has become naturalised in Tasmania and spread under native Nothofagus cunninghamii trees. A small bolete, the fruit body has a 1.6–9 cm orange-fawn cap with cinnamon to brown pores underneath, and a 4–9.5 cm high by 0.6–1.2 cm thick stipe. The flesh has a very peppery taste. The rare variety hypochryseus, found only in Europe, has yellow pores and tubes.

Serpula lacrymans is one of the fungi that cause damage to timber referred to as dry rot. It is a basidiomycete in the order Boletales. It has the ability to rapidly colonise sites through unique and highly specialised mycelium which also leads to greater degradation rates of wood cellulose.

The Dacrymycetes are a class of fungi in the Basidiomycota. The class currently contains the single order Dacrymycetales, with a second proposed order Unilacrymales now treated at the family level. The order contains four families and has a cosmopolitan distribution.

Wolfgang Steglich is a German chemist.

Pulvinone, an organic compound belonging to the esters, lactones, alcohols and butenolides classes, is a yellow crystalline solid. Although the pulvinone is not a natural product, several naturally occurring hydroxylated derivatives are known. These hydroxylated pulvinones are produced by fungal species, such as the in Europe common Larch Bolete, or by moulds such as Aspergillus terreus.

Vulpinic acid is a natural product first found in and important in the symbiosis underlying the biology of lichens. It is a simple methyl ester derivative of its parent compound, pulvinic acid, and a close relative of pulvinone, both of which derive from aromatic amino acids such as phenylalanine via secondary metabolism. The roles of vulpinic acid are not fully established, but may include properties that make it an antifeedant for herbivores. The compound is relatively toxic to mammals.

Mycena haematopus, commonly known as the bleeding fairy helmet, the burgundydrop bonnet, or the bleeding Mycena, is a species of fungus in the family Mycenaceae, of the order Agaricales. It is widespread and common in Europe and North America, and has also been collected in old Japan and Venezuela. It is saprotrophic—meaning that it obtains nutrients by consuming decomposing organic matter—and the fruit bodies appear in small groups or clusters on the decaying logs, trunks, and stumps of deciduous trees, particularly beech. The fungus, first described scientifically in 1799, is classified in the section Lactipedes of the genus Mycena, along with other species that produce a milky or colored latex.

Haematopodin is the more stable breakdown product of Haematopodin B. Both compounds are found in the mushroom Mycena haematopus, although haematopodin only occurs in trace amounts in fresh fruit bodies. Similar pigments, known as batzellins and damirones, have been found in sea sponges. A chemical synthesis for haematopodin was reported in 1996. Key steps in the synthesis involved the addition of 3-[(2,4-dimethoxybenzyl)amino]-1-propanol to the indolo-6,7-quinone and cyclization of the resulting adduct with trifluoroacetic acid.

Gyroporus cyanescens, commonly known as the bluing bolete or the cornflower bolete, is a species of bolete fungus in the family Gyroporaceae. First described from France in 1788, the species is found in Asia, Australia, Europe, and eastern North America, where it grows on the ground in coniferous and mixed forests.

Boletus curtisii is a species of fungus in the family Boletaceae. It produces small- to medium-sized fruit bodies (mushrooms) with a convex cap up to 9.5 cm (3.7 in) wide atop a slender stem that can reach a length of 12 cm (4.7 in). In young specimens, the cap and stem are bright golden yellow, although the color dulls to brownish when old. Both the stem and cap are slimy or sticky when young. On the underside of the cap are small circular to angular pores. The mushroom is edible, but not appealing. It is found in eastern and southern North America, where it grows in a mycorrhizal association with hardwood and conifer trees. Once classified as a species of Pulveroboletus, the yellow color of B. curtisii is a result of pigments chemically distinct from those responsible for the yellow coloring of Pulveroboletus.
Boletocrocin is any one of a group of seven closely related organic compounds, individually named boletocrocin A through boletrocrocin G. These compounds are polyene dicarboxylic acids that include both lipophilic and polar amino acids. They were extracted from the brightly colored mushrooms Boletus laetissimus and B. rufoaureus. The boletocrocins' conjugated systems account for the intense color.
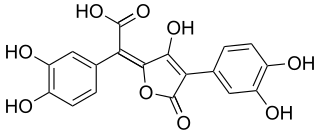
Variegatic acid is an orange pigment found in some mushrooms. It is responsible for the bluing reaction seen in many bolete mushrooms when they are injured. When mushroom tissue containing variegatic acid is exposed to air, the chemical is enzymatically oxidized to blue quinone methide anions, specifically chinonmethid anions. It is derived from xerocomic acid, which is preceded by atromentic acid and atromentin, and its genetic basis is unknown. In its oxidized form is variegatorubin, similar to xerocomorubin.

Pulvinic acids are natural chemical pigments found in some lichens, derived biosynthetically from the aromatic amino acids phenylalanine and tyrosine, via dimerization and oxidative ring-cleavage of arylpyruvic acids, a process that also produces the related pulvinones.
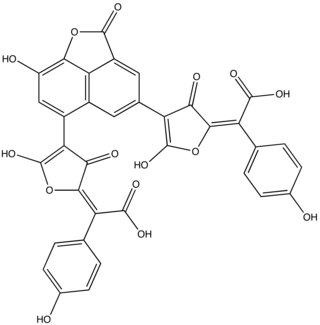
Norbadione A is a pigment found in the bay bolete mushroom. A polyphenol, norbadione A is related to a family of mushroom pigments known as pulvinic acids. The molecule has also been reported as a potassium salt from the mushrooms Pisolithus tinctorius and Chalciporus piperatus.

Imleria badia, commonly known as the bay bolete, is an edible, pored mushroom found in Eurasia and North America, where it grows in coniferous or mixed woods on the ground or on decaying tree stumps, sometimes in prolific numbers. Both the common and scientific names refer to the bay- or chestnut-coloured cap, which is almost spherical in young specimens before broadening and flattening out to a diameter up to 15 cm (6 in). On the cap underside are small yellowish pores that turn dull blue-grey when bruised. The smooth, cylindrical stipe, measuring 4–9 cm long by 1–2 cm thick, is coloured like the cap, but paler. Some varieties have been described from eastern North America, differing from the main type in both macroscopic and microscopic morphology.
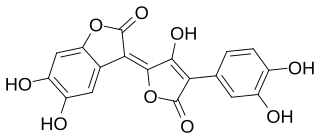
Variegatorubin is a pulvinic acid derivative. It is a red pigment that is present in many members of the Boletales, an order of the division Basidiomycota. It is generated from the oxidation of variegatic acid. Bolete species that contain variegatorubin include Neoboletus luridiformis, Chalciporus piperatus, Rhizopogon roseolus, Exsudoporus frostii, Suillellus luridus, Rubroboletus rhodoxanthus, and R. satanas. Variegatorubin was discovered by Wolfgang Steglich and colleagues, and described as a new compound in 1970.

Atromentic acid is a red-organge pigment found in fungi within the Boletales group. It is the precursor to variegatic acid and xerocomic acid, and is preceded by atromentin. As an example, it is isolated from Serpula lacrymans. It is soluble in methanol. Variants include homoatromentic acid. This pigment has been studied and elucidated by Wolfgang Steglich and colleagues over decades. When atromentin is oxidised with hydrogen peroxide a yellow product is produced. A sodium hydroxide solution is also yellow, but when this is neutralized with acid the red atromentic acid crystallises. Concentrated potassium hydroxide breaks up the compound to p-hydroxyphenylacetic acid and oxalic acid.

Xerocomorubin is a pigment from the fungus order Boletales. It is the oxidized form of isoxerocomic acid. Air oxidation is responsible its formation, and it oxidizes faster to a similar pulvinic acid type pigment oxidized variant, variegatorubin. The long wavelength has an absorption at 497 nm, 106 nm higher than its precursor isoxerocomic acid. Synthesis experiments have shown tetra-acetylation by acetic anhydride and sulfuric acid. Although xerocomorubin and variegatorubin give off the same deep red color and could simultaneously occur in a mushroom, extracts from the deep red colored mushroom Boletus rubellus Krombh. identified only variegatorubin by thin layer chromatography (TLC), leading to the question the natural abundance of xerocomorubin.



















