
Alois Alzheimer was a German psychiatrist, neuropathologist and colleague of Emil Kraepelin. He is credited with identifying the first published case of "presenile dementia", which Kraepelin later identified as Alzheimer's disease.

Dementia is a syndrome associated with many neurodegenerative diseases, characterized by a general decline in cognitive abilities that affects a person's ability to perform everyday activities. This typically involves problems with memory, thinking, behavior, and motor control. Aside from memory impairment and a disruption in thought patterns, the most common symptoms of dementia include emotional problems, difficulties with language, and decreased motivation. The symptoms may be described as occurring in a continuum over several stages. Dementia ultimately has a significant effect on the individual, their caregivers, and their social relationships in general. A diagnosis of dementia requires the observation of a change from a person's usual mental functioning and a greater cognitive decline than might be caused by the normal aging process.

Binswanger's disease, also known as subcortical leukoencephalopathy and subcortical arteriosclerotic encephalopathy, is a form of small-vessel vascular dementia caused by damage to the white brain matter. White matter atrophy can be caused by many circumstances including chronic hypertension as well as old age. This disease is characterized by loss of memory and intellectual function and by changes in mood. These changes encompass what are known as executive functions of the brain. It usually presents between 54 and 66 years of age, and the first symptoms are usually mental deterioration or stroke.

Lewy bodies are the inclusion bodies – abnormal aggregations of protein – that develop inside neurons affected by Parkinson's disease (PD), the Lewy body dementias, and some other disorders. They are also seen in cases of multiple system atrophy, particularly the parkinsonian variant (MSA-P).

Frontotemporal dementia (FTD), also called frontotemporal degeneration disease or frontotemporal neurocognitive disorder, encompasses several types of dementia involving the progressive degeneration of the brain's frontal and temporal lobes. Men and women appear to be equally affected. FTD generally presents as a behavioral or language disorder with gradual onset. Signs and symptoms tend to appear in late adulthood, typically between the ages of 45 and 65, although it can affect people younger or older than this. Currently, no cure or approved symptomatic treatment for FTD exists, although some off-label drugs and behavioral methods are prescribed.

Amyloid plaques are extracellular deposits of amyloid beta (Aβ) protein that present mainly in the grey matter of the brain. Degenerative neuronal elements and an abundance of microglia and astrocytes can be associated with amyloid plaques. Some plaques occur in the brain as a result of aging, but large numbers of plaques and neurofibrillary tangles are characteristic features of Alzheimer's disease. The plaques are highly variable in shape and size; in tissue sections immunostained for Aβ, they comprise a log-normal size distribution curve, with an average plaque area of 400-450 square micrometers (μm2). The smallest plaques, which often consist of diffuse deposits of Aβ, are particularly numerous. Plaques form when Aβ misfolds and aggregates into oligomers and longer polymers, the latter of which are characteristic of amyloid.
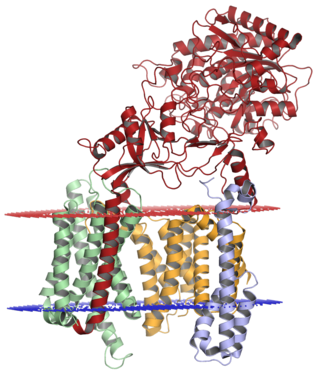
Gamma secretase is a multi-subunit protease complex, an integral membrane protein, that cleaves single-pass transmembrane proteins at residues within the transmembrane domain. Proteases of this type are known as intramembrane proteases. The most well-known substrate of gamma secretase is amyloid precursor protein, a large integral membrane protein that, when cleaved by both gamma and beta secretase, produces a short 37-43 amino acid peptide called amyloid beta whose abnormally folded fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. Gamma secretase is also critical in the related processing of several other type I integral membrane proteins, such as Notch, ErbB4, E-cadherin, N-cadherin, ephrin-B2, or CD44.

Sortilin-related receptor, L(DLR class) A repeats containing is a protein that in humans is encoded by the SORL1 gene.

Nicastrin, also known as NCSTN, is a protein that in humans is encoded by the NCSTN gene.
Bart De Strooper is a Belgian molecular biologist and professor at Vlaams Instituut voor Biotechnologie and KU Leuven and the UK Dementia Research Institute and University College London, UK. De Strooper's research seeks to translate genetic data into the identification and treatment of neurodegenerative diseases and treatments. interest are the secretases, proteases which cleave the amyloid precursor protein (APP), resulting in amyloid peptides.

Presenilin-1(PS-1) is a presenilin protein that in humans is encoded by the PSEN1 gene. Presenilin-1 is one of the four core proteins in the gamma secretase complex, which is considered to play an important role in generation of amyloid beta (Aβ) from amyloid-beta precursor protein (APP). Accumulation of amyloid beta is associated with the onset of Alzheimer's disease.

Presenilin-2 is a protein that is encoded by the PSEN2 gene.

Solomon Carter Fuller was a pioneering Liberian neurologist, psychiatrist, pathologist, and professor. Born in Monrovia, Liberia, he completed his college education and medical degree (MD) in the United States. He studied psychiatry in Munich, Germany, then returned to the United States, where he worked for much of his career at Westborough State Hospital in Westborough, Massachusetts.

Posterior cortical atrophy (PCA), also called Benson's syndrome, is a rare form of dementia which is considered a visual variant or an atypical variant of Alzheimer's disease (AD). The disease causes atrophy of the posterior part of the cerebral cortex, resulting in the progressive disruption of complex visual processing. PCA was first described by D. Frank Benson in 1988.

Gaetano Perusini was an Italian physician. He was the pupil and colleague of Alois Alzheimer and contributed to the definition of Alzheimer's disease.

Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens. It is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems with language, disorientation, mood swings, loss of motivation, self-neglect, and behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the average life expectancy following diagnosis is three to twelve years.
Early-onset Alzheimer's disease (EOAD), also called younger-onset Alzheimer's disease (YOAD), is Alzheimer's disease diagnosed before the age of 65. It is an uncommon form of Alzheimer's, accounting for only 5–10% of all Alzheimer's cases. About 60% have a positive family history of Alzheimer's and 13% of them are inherited in an autosomal dominant manner. Most cases of early-onset Alzheimer's share the same traits as the "late-onset" form and are not caused by known genetic mutations. Little is understood about how it starts.
Peter Henry St George-Hyslop is a British and Canadian medical scientist, neurologist and molecular geneticist who is known for his research into neurodegenerative diseases. St George-Hyslop is one of the most cited authors in the field of Alzheimer's disease research. He has identified a number of key genes that are responsible for nerve cell degeneration and early-onset forms of Alzheimer's disease. These include the discovery of the presenilins, Nicastrin, TREM2, Apolipoprotein E and SORL1 genes. Presenilin mutations are the most common cause of familial Alzheimer's disease. St George-Hyslop also co-led the discovery of the gene for the amyloid precursor protein.
Alzheimer's disease (AD) in the Hispanic/Latino population is becoming a topic of interest in AD research as Hispanics and Latinos are disproportionately affected by Alzheimer's Disease and underrepresented in clinical research. AD is a neurodegenerative disease, characterized by the presence of amyloid-beta plaques and neurofibrillary tangles, that causes memory loss and cognitive decline in its patients. However, pathology and symptoms have been shown to manifest differently in Hispanic/Latinos, as different neuroinflammatory markers are expressed and cognitive decline is more pronounced. Additionally, there is a large genetic component of AD, with mutations in the amyloid precursor protein (APP), apolipoprotein E APOE), presenilin 1 (PSEN1), bridging Integrator 1 (BIN1), SORL1, and clusterin (CLU) genes increasing one's risk to develop the condition. However, research has shown these high-risk genes have a different effect on Hispanics and Latinos then they do in other racial and ethnic groups. Additionally, this population experiences higher rates of comorbidities, that increase their risk of developing AD. Hispanics and Latinos also face socioeconomic and cultural factors, such as low income and a language barrier, that affect their ability to engage in clinical trials and receive proper care.
Alzheimer's disease (AD) is a complex neurodegenerative disease that affects millions of people across the globe. It is also a topic of interest in the East Asian population, especially as the burden of disease increases due to aging and population growth. The pathogenesis of AD between ethnic groups is different. However, prior studies in AD pathology have focused primarily on populations of European ancestry and may not give adequate insight on the genetic, clinical, and biological differences found in East Asians with AD. Gaps in knowledge regarding Alzheimer's disease in the East Asian population introduce serious barriers to screening, early prevention, diagnosis, treatment, and timely intervention.














