
Flavan-3-ols are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. These compounds include catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins.

Catechin is a flavan-3-ol, a type of natural phenol and antioxidant. It is a plant secondary metabolite. It belongs to the group of flavan-3-ols, part of the chemical family of flavonoids.
Proanthocyanidins are a class of polyphenols found in a variety of plants such as blueberry. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Trimethylsilyl trifluoromethanesulfonate is a trifluoromethanesulfonate derivate with a trimethylsilyl R-group. It has similar reactivity to trimethylsilyl chloride, and is also used often in organic synthesis.
Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Procyanidin C2 is a B type proanthocyanidin trimer, a type of condensed tannin.

Procyanidin B2 is a B type proanthocyanidin. Its structure is (−)-Epicatechin-(4β→8)-(−)-epicatechin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.
The molecular formula C30H26O12 may refer to:

Procyanidin B1 is a procyanidin dimer.
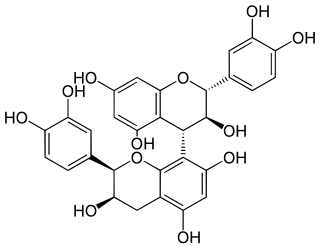
Procyanidin B4 is a B type proanthocyanidin.

Procyanidin B5 is a B type proanthocyanidin.

Procyanidin B6 is a B type proanthocyanidin.
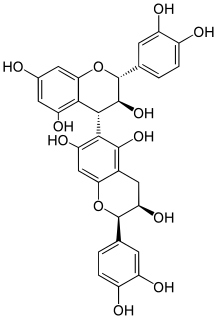
Procyanidin B8 is a B type proanthocyanidin.
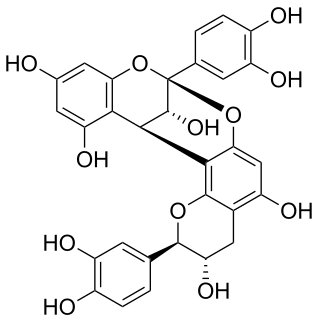
Procyanidin A1 is an A type proanthocyanidin dimer.

Procyanidin C1 is a B type proanthocyanidin. It is an epicatechin trimer found in grape.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.

Gambier or gambir is an extract derived from the leaves of Uncaria gambir, a climbing shrub native to tropical Southeast Asia. Gambier is produced in Indonesia and Malaysia where it was an important trade item into the late nineteenth century. It can be used as a tanning agent, a brown dye, a food additive and as herbal medicine. Also known as pale catechu, white catechu or Japan Earth, it is often confused with other forms of catechu.

















