
Flavan-3-ols are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.

Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids.
Proanthocyanidins are a class of polyphenols found in many plants, such as cranberry, blueberry, and grape seeds. Chemically, they are oligomeric flavonoids. Many are oligomers of catechin and epicatechin and their gallic acid esters. More complex polyphenols, having the same polymeric building block, form the group of tannins.

Procyanidins are members of the proanthocyanidin class of flavonoids. They are oligomeric compounds, formed from catechin and epicatechin molecules. They yield cyanidin when depolymerized under oxidative conditions.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Prodelphinidin is a name for the polymeric tannins composed of gallocatechin. It yields delphinidin during depolymerisation under oxidative conditions.

Prodelphinidin B3 is a prodelphinidin dimer found in food products such as barley and beer, in fruits and pod vegetables. It can also be found in pomegranate peels.

Leucocyanidin is a colorless chemical compound that is a member of the class of natural products known as leucoanthocyanidins.

Oenin is an anthocyanin. It is the 3-glucoside of malvidin. It is one of the red pigments found in the skin of purple grapes and in wine.

Procyanidin B2 is a B type proanthocyanidin. Its structure is (−)-Epicatechin-(4β→8)-(−)-epicatechin.
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.

Procyanidin B1 is a procyanidin dimer.
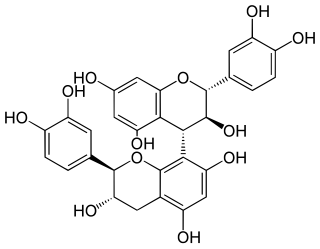
Procyanidin B3 is a B type proanthocyanidin. Procyanidin B3 is a catechin dimer.
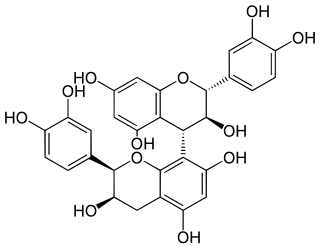
Procyanidin B4 is a B type proanthocyanidin.

Procyanidin B5 is a B type proanthocyanidin.
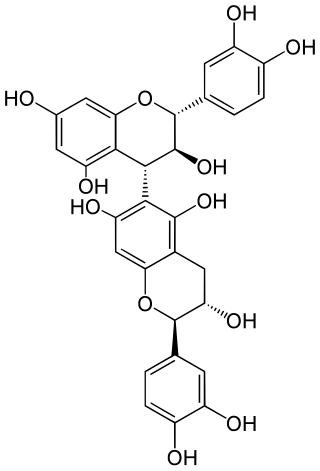
Procyanidin B6 is a B type proanthocyanidin.
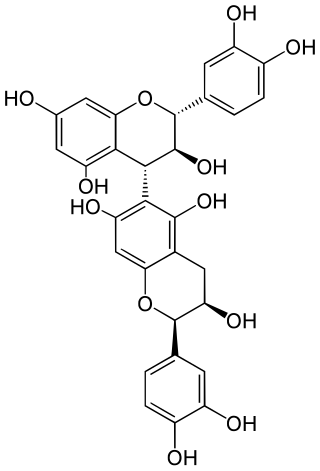
Procyanidin B8 is a B type proanthocyanidin.

Procyanidin C1 (PCC1) is a B type proanthocyanidin. It is an epicatechin trimer found in grape, unripe apples, and cinnamon.

Condensed tannins are polymers formed by the condensation of flavans. They do not contain sugar residues.
B type proanthocyanidins are a specific type of proanthocyanidin, which are a class of flavanoids. They are oligomers of flavan-3-ols.
















