
Phenylalanine hydroxylase (PAH) (EC 1.14.16.1) is an enzyme that catalyzes the hydroxylation of the aromatic side-chain of phenylalanine to generate tyrosine. PAH is one of three members of the biopterin-dependent aromatic amino acid hydroxylases, a class of monooxygenase that uses tetrahydrobiopterin (BH4, a pteridine cofactor) and a non-heme iron for catalysis. During the reaction, molecular oxygen is heterolytically cleaved with sequential incorporation of one oxygen atom into BH4 and phenylalanine substrate.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Papain, also known as papaya proteinase I, is a cysteine protease enzyme present in papaya and mountain papaya.
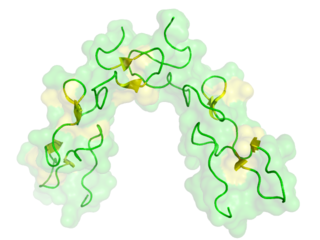
Disintegrins are a family of small proteins from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion.
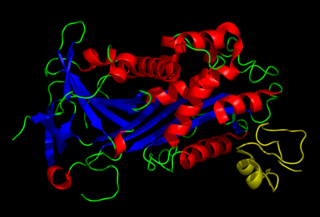
Vitronectin is a glycoprotein of the hemopexin family which is abundantly found in serum, the extracellular matrix and bone. In humans it is encoded by the VTN gene.
Carbamoyl phosphate synthetase I is a ligase enzyme located in the mitochondria involved in the production of urea. Carbamoyl phosphate synthetase I transfers an ammonia molecule from glutamine or glutamate to a molecule of bicarbonate that has been phosphorylated by a molecule of ATP. The resulting carbamate is then phosphorylated with another molecule of ATP. The resulting molecule of carbamoyl phosphate leaves the enzyme.

Diphtheria toxin is an exotoxin secreted by Corynebacterium, the pathogenic bacterium that causes diphtheria. The toxin gene is encoded by a prophage. The toxin causes the disease in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.

The acetolactate synthase (ALS) enzyme is a protein found in plants and micro-organisms. ALS catalyzes the first step in the synthesis of the branched-chain amino acids.

Leucyl aminopeptidases are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, Escherichia coli LAP, and the solanaceous-specific acidic LAP (LAP-A) in tomato.

Carbamoyl phosphate synthetase catalyzes the ATP-dependent synthesis of carbamoyl phosphate from glutamine or ammonia and bicarbonate. This enzyme catalyzes the reaction of ATP and bicarbonate to produce carboxy phosphate and ADP. Carboxy phosphate reacts with ammonia to give carbamic acid. In turn, carbamic acid reacts with a second ATP to give carbamoyl phosphate plus ADP.

Myocilin, trabecular meshwork inducible glucocorticoid response, also known as MYOC, is a protein which in humans is encoded by the MYOC gene. Mutations in MYOC are a major cause of glaucoma.

Acid-Induced Arginine Decarboxylase (AdiA), also commonly referred to as arginine decarboxylase, is an enzyme responsible for catalyzing the conversion of L-arginine into agmantine and carbon dioxide. The process consumes a proton in the decarboxylation and employs a pyridoxal-5'-phosphate (PLP) cofactor, similar to other enzymes involved in amino acid metabolism, such as ornithine decarboxylase and glutamine decarboxylase. It is found in bacteria and virus, though most research has so far focused on forms of the enzyme in bacteria. During the AdiA catalyzed decarboxylation of arginine, the necessary proton is consumed from the cell cytoplasm which helps to prevent the over-accumulation of protons inside the cell and serves to increase the intracellular pH. Arginine decarboxylase is part of an enzymatic system in Escherichia coli , Salmonella typhimurium, and methane-producing bacteria Methanococcus jannaschii that makes these organisms acid resistant and allows them to survive under highly acidic medium.

A cutinase (EC 3.1.1.74) is an enzyme that catalyzes the chemical reaction
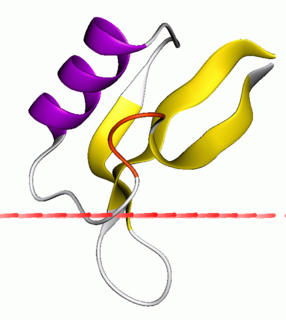
Plant defensins are a family of small, cysteine-rich proteins found in plants that serve to defend them against parasites.
Streptogramin B is a subgroup of the streptogramin antibiotics family. These natural products are cyclic hexa- or hepta depsipeptides produced by various members of the genus of bacteria Streptomyces. Many of the members of the streptogramins reported in the literature have the same structure and different names; for example, pristinamycin IA = vernamycin Bα = mikamycin B = osteogrycin B.

In molecular biology, the amylin protein family or calcitonin/CGRP/IAPP protein family' is a family of proteins, which includes the precursors of calcitonin/calcitonin gene-related peptide (CGRP), islet amyloid polypeptide (IAPP) and adrenomedullin.

In molecular biology, the carboxypeptidase A inhibitor family is a family of proteins which is represented by the well-characterised metallocarboxypeptidase A inhibitor (MCPI) from potatoes, which belongs to the MEROPS inhibitor family I37, clan IE. It inhibits metallopeptidases belonging to MEROPS peptidase family M14, carboxypeptidase A. In Russet Burbank potatoes, it is a mixture of approximately equal amounts of two polypeptide chains containing 38 or 39 amino acid residues. The chains differ in their amino terminal sequence only and are resistant to fragmentation by proteases. The structure of the complex between bovine carboxypeptidase A and the 39-amino-acid carboxypeptidase A inhibitor from potatoes has been determined at 2.5-Angstrom resolution.

In molecular biology the FGGY carbohydrate kinase family is a family of evolutionary related carbohydrate kinase enzymes. These enzymes include L-fucolokinase EC 2.7.1.51 ; gluconokinase EC 2.7.1.12 ; glycerol kinase EC 2.7.1.30 ; xylulokinase EC 2.7.1.17 ; D-ribulose kinase EC 2.7.1.47 ; and L-xylulose kinase EC 2.7.1.53. These enzymes are proteins of from 480 to 520 amino acid residues.

Pro-hevein is a wound-induced and a lectin-like protein from Hevea brasiliensis where it is involved in the coagulation of latex.
Hemp protein is the protein content of hemp seeds. The protein in hemp seeds is made up of the two globulin types of proteins, edestin (60–80%) and albumin, with edestin also being rich in the essential amino acids. Hemp protein has a PDCAAS score of 0.61 and a biological value of 87. The total proportion of essential amino acids in hemp protein isolate, is also significantly higher than that of soy protein isolate.



















