Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen. The heteroatom may vary, but it usually is oxygen, nitrogen, or sulfur. A related reaction is hydrogenation, where hydrogen is added to the molecule, without cleaving bonds. Usually hydrogenolysis is conducted catalytically using hydrogen gas.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C6H5CH2–. Benzyl features a benzene ring attached to a CH2 group.
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures without assumptions regarding starting materials. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. E.J. Corey formalized this concept in his book The Logic of Chemical Synthesis.

Phenylacetic acid (PAA), also known by various synonyms, is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a strong honey-like odor. Endogeneously, it is a catabolite of phenylalanine. As a commercial chemical, because it can be used in the illicit production of phenylacetone, it is subject to controls in countries including the United States and China.
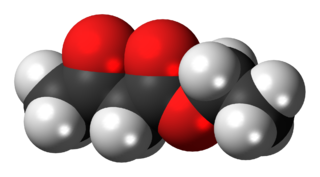
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and aminopyrine, and vitamin B1; as well as the manufacture of dyes, inks, lacquers, perfumes, plastics, and yellow paint pigments. Alone, it is used as a flavoring for food.

Benzyl acetate is an organic ester with the molecular formula C9H10O2. It is formed by the condensation of benzyl alcohol and acetic acid.
Benzyl bromide is an organic compound with the formula C6H5CH2Br. The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties. The compound is a reagent for introducing benzyl groups.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colourless liquid is a reactive organochlorine compound that is a widely used chemical building block.
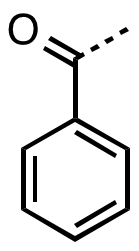
In organic chemistry, benzoyl (, BENZ-oh-il) is the functional group with the formula C6H5CO-.

Diphenylmethane is an organic compound with the formula (C6H5)2CH2. The compound consists of methane wherein two hydrogen atoms are replaced by two phenyl groups. Diphenylmethane forms a common skeleton in organic chemistry; the diphenylmethyl group is also known as benzhydryl.
Benzal chloride is an organic compound with the formula C6H5CHCl2. This colourless liquid is a lachrymator and is used as a building block in organic synthesis.

Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry.
Organochromium chemistry is a branch of organometallic chemistry that deals with organic compounds containing a chromium to carbon bond and their reactions. The field is of some relevance to organic synthesis. The relevant oxidation states for chromium range from -2 to +6.
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.

Benzyl mercaptan is an organosulfur compound with the formula C6H5CH2SH. It is a common laboratory alkylthiol that occurs in trace amounts naturally. It is a colorless, malodorous liquid.

Veratrole alcohol is an organic compound related to veratrole and also to benzyl alcohol. It can be obtained by reduction of veratraldehyde. Veratrole alcohol is the raw material for the synthesis of cyclotriveratrylene which is used in host–guest chemistry. It is a secondary metabolite of some white rot fungi and is believed to play a role in their degradation of lignin.

Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The electron withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis.

Benzyl cinnamate is the chemical compound which is the ester derived from cinnamic acid and benzyl alcohol.

Benzyl iodide is an organic compound with the chemical formula C
7H
7I. The compound consists of a benzene ring with an attached iodidemethyl group. The substance is an alkyl halide and is a constitutional isomer of the iodotoluenes.

n-Propylbenzene is an aromatic hydrocarbon with the formula C6H5CH2CH2CH3. The molecule consisting of a propyl group attached to a phenyl ring. It is a colorless liquid. A more common structural isomer of this compound is cumene.