Research
Bergaptol is researched for anti-inflammatory, antioxidant, anti-cancer, anti-osteoporosis, anti-microbial, and anti-lipidemic properties. [2]
Bergaptol inhibits cytochrome P450 enzymes (CYP), particularly CYP2C9 and CYP3A4. This inhibition can impact the metabolism and concentrations of various drugs and toxins within the body. When compared to other coumarins, bergaptol demonstrates the lowest potency in inhibiting CYP3A4 specifically in cancer cells. Instead, it has the ability to suppress drug efflux transporters, such as P-glycoprotein, which can help overcome chemotherapeutic drug resistance. [2]
Bergaptol exhibits strong antimicrobial effects and has a high potential for inhibiting quorum sensing, a process crucial for microbial communication and virulence. [2]
In vivo studies have shown that bergaptol has a longer retention time in plasma compared to other coumarins. [2]
Toxicity of bergaptol in humans has not been extensively studied and remains unclear. [2]

The grapefruit is a subtropical citrus tree known for its relatively large, sour to semi-sweet, somewhat bitter fruit. The interior flesh is segmented and varies in color from pale yellow to dark pink/red.
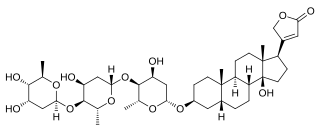
Digitoxin is a cardiac glycoside used for the treatment of heart failure and certain kinds of heart arrhythmia. It is a phytosteroid and is similar in structure and effects to digoxin, though the effects are longer-lasting. Unlike digoxin, which is eliminated from the body via the kidneys, it is eliminated via the liver, and so can be used in patients with poor or erratic kidney function. While several controlled trials have shown digoxin to be effective in a proportion of patients treated for heart failure, the evidence base for digitoxin is not as strong, although it is presumed to be similarly effective.
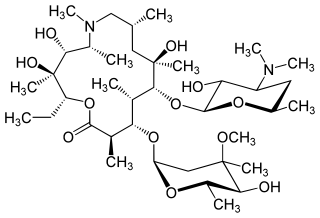
Azithromycin, sold under the brand names Zithromax and Azasite, is an antibiotic medication used for the treatment of several bacterial infections. This includes middle ear infections, strep throat, pneumonia, traveler's diarrhea, and certain other intestinal infections. Along with other medications, it may also be used for malaria. It is administered by mouth, into a vein, or into the eye.

Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine, which in humans is encoded by CYP3A4 gene. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme.
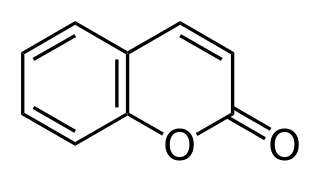
Coumarin or 2H-chromen-2-one is an aromatic organic chemical compound with formula C9H6O2. Its molecule can be described as a benzene molecule with two adjacent hydrogen atoms replaced by an unsaturated lactone ring −(CH)=(CH)−(C=O)−O−, forming a second six-membered heterocycle that shares two carbons with the benzene ring. It belongs to the benzopyrone chemical class and considered as a lactone.

An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid; thus, competitive inhibition can occur, and the presence of antimetabolites can have toxic effects on cells, such as halting cell growth and cell division, so these compounds are used in chemotherapy for cancer.

Naringin is a flavanone-7-O-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness (debittering) created by naringin. In humans naringin is metabolized to the aglycone naringenin by naringinase present in the gut.
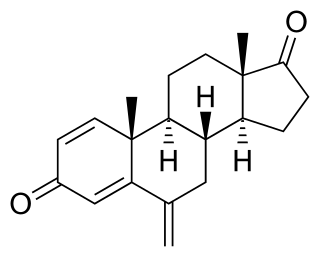
Exemestane, sold under the brand name Aromasin among others, is a medication used to treat breast cancer. It is a member of the class of antiestrogens known as aromatase inhibitors. Some breast cancers require estrogen to grow. Those cancers have estrogen receptors (ERs), and are called ER-positive. They may also be called estrogen-responsive, hormonally-responsive, or hormone-receptor-positive. Aromatase is an enzyme that synthesizes estrogen. Aromatase inhibitors block the synthesis of estrogen. This lowers the estrogen level, and slows the growth of cancers.
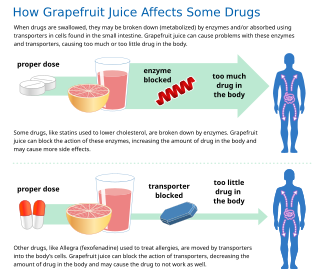
Some fruit juices and fruits can interact with numerous drugs, in many cases causing adverse effects. The effect is most studied with grapefruit and grapefruit juice, but similar effects have been observed with certain other citrus fruits.

In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targets to which the drug binds, such as an enzyme or receptor. Receptor sites have specific affinities for drugs based on the chemical structure of the drug, as well as the specific action that occurs there.

Phenprocoumon is a long-acting blood thinner drug to be taken by mouth, and a coumarin derivative. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.

Bergamottin (5-geranoxypsoralen) is a natural furanocoumarin found in the pulp of pomelos and grapefruits. It is also found in the peel and pulp of the bergamot orange, from which it was first isolated and from which its name is derived.

A mitotic inhibitor, microtubule inhibitor, or tubulin inhibitor, is a drug that inhibits mitosis, or cell division, and is used in treating cancer, gout, and nail fungus. These drugs disrupt microtubules, which are structures that pull the chromosomes apart when a cell divides. Mitotic inhibitors are used in cancer treatment, because cancer cells are able to grow through continuous division that eventually spread through the body (metastasize). Thus, cancer cells are more sensitive to inhibition of mitosis than normal cells. Mitotic inhibitors are also used in cytogenetics, where they stop cell division at a stage where chromosomes can be easily examined.

Bergapten (5-methoxypsoralen) is a naturally-occurring organic chemical compound produced by numerous plant species, especially from the carrot family Apiaceae and the citrus family Rutaceae. For example, bergapten has been extracted from 24 species of the genus Heracleum in the family Apiaceae. In the family Rutaceae, various Citrus species contain significant amounts of bergapten, especially the bergamot orange, the micrantha, and certain varieties of lime and bitter orange.

Daphnin is a plant toxin with the chemical formula C15H16O9 and is one of the active compounds present in the Eurasian and North African genus Daphne of the Thymelaeaceae, a plant family with a predominantly Southern Hemisphere distribution with concentrations in Australia and tropical Africa.

Angelicin is the parent compound in a family of naturally occurring organic compounds known as the angular furanocoumarins. Structurally, it can be considered as benzapyra-2-one fused with a furan moiety in the 7,8-position. Angelicin is commonly found in certain Apiaceae and Fabaceae plant species such as Bituminaria bituminosa. It has a skin permeability coefficient (LogKp) of -2.46. The maximum absorption is observed at 300 nm. The 1HNMR spectrum is available; the infrared and mass spectra of angelicin can be found in this database. The sublimation of angelicin occurs at 120 °C and the pressure of 0.13 Pa. Angelicin is a coumarin.

Ribociclib, sold under the brand name Kisqali, is a medication used for the treatment of certain kinds of breast cancer. Ribociclib is a kinase inhibitor. It was developed by Novartis and Astex Pharmaceuticals.

Lorlatinib, sold under the brand name Lorbrena in the United States, Canada, and Japan, and Lorviqua in the European Union, is an anti-cancer medication used for the treatment of non-small cell lung cancer. It is an orally administered inhibitor of anaplastic lymphoma kinase (ALK) and C-ros oncogene 1 (ROS1), two enzymes that play a role in the development of cancer. It was developed by Pfizer.
VEGFR-2 inhibitor, also known as kinase insert domain receptor(KDR) inhibitor, are tyrosine kinase receptor inhibitors that reduce angiogenesis or lymphangiogenesis, leading to anticancer activity. Generally they are small, synthesised molecules that bind competitively to the ATP-site of the tyrosine kinase domain. VEGFR-2 selective inhibitor can interrupt multiple signaling pathways involved in tumor, including proliferation, metastasis and angiogenesis.


















