Related Research Articles

Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18
F
-FDG is commonly used to detect cancer, NaF18
F
is widely used for detecting bone formation, and oxygen-15 is sometimes used to measure blood flow.

Medical imaging is the technique and process of imaging the interior of a body for clinical analysis and medical intervention, as well as visual representation of the function of some organs or tissues (physiology). Medical imaging seeks to reveal internal structures hidden by the skin and bones, as well as to diagnose and treat disease. Medical imaging also establishes a database of normal anatomy and physiology to make it possible to identify abnormalities. Although imaging of removed organs and tissues can be performed for medical reasons, such procedures are usually considered part of pathology instead of medical imaging.

Single-photon emission computed tomography is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera, but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required.

Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is "radiology done inside out" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine.
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, use of radioisotope tracers are sometimes called radioisotope feeding experiments.

Scintigraphy, also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and the emitted gamma radiation is captured by external detectors to form two-dimensional images in a similar process to the capture of x-ray images. In contrast, SPECT and positron emission tomography (PET) form 3-dimensional images and are therefore classified as separate techniques from scintigraphy, although they also use gamma cameras to detect internal radiation. Scintigraphy is unlike a diagnostic X-ray where external radiation is passed through the body to form an image.

Perfusion is the passage of fluid through the circulatory system or lymphatic system to an organ or a tissue, usually referring to the delivery of blood to a capillary bed in tissue. Perfusion is measured as the rate at which blood is delivered to tissue, or volume of blood per unit time per unit tissue mass. The SI unit is m3/(s·kg), although for human organs perfusion is typically reported in ml/min/g. The word is derived from the French verb "perfuser" meaning to "pour over or through". All animal tissues require an adequate blood supply for health and life. Poor perfusion (malperfusion), that is, ischemia, causes health problems, as seen in cardiovascular disease, including coronary artery disease, cerebrovascular disease, peripheral artery disease, and many other conditions.
Natural gallium (31Ga) consists of a mixture of two stable isotopes: gallium-69 and gallium-71. The most commercially important radioisotopes are gallium-67 and gallium-68.

[18F]Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 2-[18F]FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.

Molecular imaging is a field of medical imaging that focuses on imaging molecules of medical interest within living patients. This is in contrast to conventional methods for obtaining molecular information from preserved tissue samples, such as histology. Molecules of interest may be either ones produced naturally by the body, or synthetic molecules produced in a laboratory and injected into a patient by a doctor. The most common example of molecular imaging used clinically today is to inject a contrast agent into a patient's bloodstream and to use an imaging modality to track its movement in the body. Molecular imaging originated from the field of radiology from a need to better understand fundamental molecular processes inside organisms in a noninvasive manner.

Neuroimaging is the use of quantitative (computational) techniques to study the structure and function of the central nervous system, developed as an objective way of scientifically studying the healthy human brain in a non-invasive manner. Increasingly it is also being used for quantitative studies of brain disease and psychiatric illness. Neuroimaging is a highly multidisciplinary research field and is not a medical specialty.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).

Radioactivity is generally used in life sciences for highly sensitive and direct measurements of biological phenomena, and for visualizing the location of biomolecules radiolabelled with a radioisotope.

Myocardial perfusion imaging or scanning is a nuclear medicine procedure that illustrates the function of the heart muscle (myocardium).
Perfusion is the passage of fluid through the lymphatic system or blood vessels to an organ or a tissue. The practice of perfusion scanning is the process by which this perfusion can be observed, recorded and quantified. The term perfusion scanning encompasses a wide range of medical imaging modalities.
Nuclear medicine physicians, also called nuclear radiologists or simply nucleologists, are medical specialists that use tracers, usually radiopharmaceuticals, for diagnosis and therapy. Nuclear medicine procedures are the major clinical applications of molecular imaging and molecular therapy. In the United States, nuclear medicine physicians are certified by the American Board of Nuclear Medicine and the American Osteopathic Board of Nuclear Medicine.
Rubidium-82 (82Rb) is a radioactive isotope of rubidium. 82Rb is widely used in myocardial perfusion imaging. This isotope undergoes rapid uptake by myocardiocytes, which makes it a valuable tool for identifying myocardial ischemia in Positron Emission Tomography (PET) imaging. 82Rb is used in the pharmaceutical industry and is marketed as Rubidium-82 chloride under the trade names RUBY-FILL and CardioGen-82.
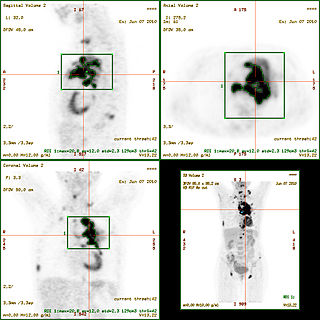
The standardized uptake value (SUV) is a nuclear medicine term, used in positron emission tomography (PET) as well as in modern calibrated single photon emission tomography (SPECT) imaging for a semiquantitative analysis. Its use is particularly common in the analysis of [18F]fluorodeoxyglucose ([18F]FDG) images of cancer patients. It can also be used with other PET agents especially when no arterial input function is available for more detailed pharmacokinetic modeling. Otherwise measures like the fractional uptake rate (FUR) or parameters from more advanced pharmacokinetic modeling may be preferable.
Preclinical imaging is the visualization of living animals for research purposes, such as drug development. Imaging modalities have long been crucial to the researcher in observing changes, either at the organ, tissue, cell, or molecular level, in animals responding to physiological or environmental changes. Imaging modalities that are non-invasive and in vivo have become especially important to study animal models longitudinally. Broadly speaking, these imaging systems can be categorized into primarily morphological/anatomical and primarily molecular imaging techniques. Techniques such as high-frequency micro-ultrasound, magnetic resonance imaging (MRI) and computed tomography (CT) are usually used for anatomical imaging, while optical imaging, positron emission tomography (PET), and single photon emission computed tomography (SPECT) are usually used for molecular visualizations.
Preclinical or small-animal Single Photon Emission Computed Tomography (SPECT) is a radionuclide based molecular imaging modality for small laboratory animals. Although SPECT is a well-established imaging technique that is already for decades in use for clinical application, the limited resolution of clinical SPECT (~10 mm) stimulated the development of dedicated small animal SPECT systems with sub-mm resolution. Unlike in clinics, preclinical SPECT outperforms preclinical coincidence PET in terms of resolution and, at the same time, allows to perform fast dynamic imaging of animals.
References
- ↑ Raty JK, Liimatainen T, Wirth T, et al. (October 2006). "Magnetic resonance imaging of viral particle biodistribution in vivo". Gene Therapy. 13 (20): 1440–46. doi: 10.1038/sj.gt.3302828 . PMID 16855615.