Related Research Articles

Gasoline or petrol is a transparent, slight yellowish petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. On average, U.S. refineries produce, from a barrel of crude oil, about 19 to 20 gallons of gasoline; 11 to 13 gallons of distillate fuel ; and 3 to 4 gallons of jet fuel. The product ratio depends on the processing in an oil refinery and the crude oil assay.

Biofuel is a fuel that is produced over a short time span from biomass, rather than by the very slow natural processes involved in the formation of fossil fuels, such as oil. Biofuel can be produced from plants or from agricultural, domestic or industrial biowaste.

In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large hydrocarbons into smaller, more useful alkanes and alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain of hydrocarbons into short ones. This process requires high temperatures.
Natural-gas condensate, also called natural gas liquids, is a low-density mixture of hydrocarbon liquids that are present as gaseous components in the raw natural gas produced from many natural gas fields. Some gas species within the raw natural gas will condense to a liquid state if the temperature is reduced to below the hydrocarbon dew point temperature at a set pressure.

Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.

Gas to liquids (GTL) is a refinery process to convert natural gas or other gaseous hydrocarbons into longer-chain hydrocarbons, such as gasoline or diesel fuel. Methane-rich gases are converted into liquid synthetic fuels. Two general strategies exist: (i) direct partial combustion of methane to methanol and (ii) Fischer–Tropsch-like processes that convert carbon monoxide and hydrogen into hydrocarbons. Strategy ii is followed by diverse methods to convert the hydrogen-carbon monoxide mixtures to liquids. Direct partial combustion has been demonstrated in nature but not replicated commercially. Technologies reliant on partial combustion have been commercialized mainly in regions where natural gas is inexpensive.

The Bergius process is a method of production of liquid hydrocarbons for use as synthetic fuel by hydrogenation of high-volatile bituminous coal at high temperature and pressure. It was first developed by Friedrich Bergius in 1913. In 1931 Bergius was awarded the Nobel Prize in Chemistry for his development of high-pressure chemistry.
Pyrolysis oil, sometimes also known as bio-crude or bio-oil, is a synthetic fuel under investigation as substitute for petroleum. It is obtained by heating dried biomass without oxygen in a reactor at a temperature of about 500 °C (900 °F) with subsequent cooling. Pyrolysis oil is a kind of tar and normally contains levels of oxygen too high to be considered a pure hydrocarbon. This high oxygen content results in non-volatility, corrosiveness, immiscibility with fossil fuels, thermal instability, and a tendency to polymerize when exposed to air. As such, it is distinctly different from petroleum products. Removing oxygen from bio-oil or nitrogen from algal bio-oil is known as upgrading.

Butanol may be used as a fuel in an internal combustion engine. It is more similar to gasoline than it is to ethanol. A C4-hydrocarbon, butanol is a drop-in fuel and thus works in vehicles designed for use with gasoline without modification. Both n-butanol and isobutanol have been studied as possible fuels. Both can be produced from biomass (as "biobutanol" ) as well as from fossil fuels (as "petrobutanol"). The chemical properties depend on the isomer (n-butanol or isobutanol), not on the production method.
Renewable Fuels are fuels produced from renewable resources. Examples include: biofuels, Hydrogen fuel, and fully synthetic fuel produced from ambient carbon dioxide and water. This is in contrast to non-renewable fuels such as natural gas, LPG (propane), petroleum and other fossil fuels and nuclear energy. Renewable fuels can include fuels that are synthesized from renewable energy sources, such as wind and solar. Renewable fuels have gained in popularity due to their sustainability, low contributions to the carbon cycle, and in some cases lower amounts of greenhouse gases. The geo-political ramifications of these fuels are also of interest, particularly to industrialized economies which desire independence from Middle Eastern oil.

Botryococcus braunii is a green, pyramid-shaped planktonic microalga that is of potentially great importance in the field of biotechnology. Colonies held together by a lipid biofilm matrix can be found in temperate or tropical oligotrophic lakes and estuaries, and will bloom when in the presence of elevated levels of dissolved inorganic phosphorus. The species is notable for its ability to produce high amounts of hydrocarbons, especially oils in the form of Triterpenes, that are typically around 30–40% of their dry weight. Compared to other green alge species it has a relatively thick cell wall that is accumulated from previous cellular divisions; making extraction of cytoplasmic components rather difficult. Much of the useful hydrocarbon oil is outside of the cell.
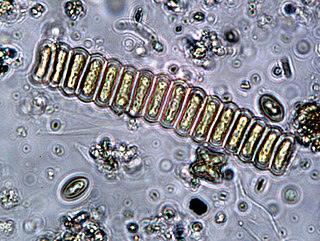
Scenedesmus is a genus of green algae, in the class Chlorophyceae. They are colonial and non-motile. They are one of the most common components of phytoplankton in freshwater habitats worldwide.
Second-generation biofuels, also known as advanced biofuels, are fuels that can be manufactured from various types of non-food biomass. Biomass in this context means plant materials and animal waste used especially as a source of fuel.

Botryococcus is a genus of green algae. The cells form an irregularly shaped aggregate. Thin filaments connect the cells. The cell body is ovoid, 6 to 10 μm long, and 3 to 6 μm wide. Fossils of the genus are known since Precambrian times, and form the single largest biological contributor to crude oil, and are a major component of oil shales.

Algae fuel, algal biofuel, or algal oil is an alternative to liquid fossil fuels that uses algae as its source of energy-rich oils. Also, algae fuels are an alternative to commonly known biofuel sources, such as corn and sugarcane. When made from seaweed (macroalgae) it can be known as seaweed fuel or seaweed oil.
Hydrotreated vegetable oil (HVO) is a biofuel made by the hydrocracking or hydrogenation of vegetable oil. Hydrocracking breaks big molecules into smaller ones using hydrogen while hydrogenation adds hydrogen to molecules. These methods can be used to create substitutes for gasoline, diesel, propane, kerosene and other chemical feedstock. Diesel fuel produced from these sources is known as green diesel or renewable diesel.
Biogasoline, or biopetrol, is a type of gasoline produced from biomass such as algae. Like traditionally produced gasoline, it is made up of hydrocarbons with 6 (hexane) to 12 (dodecane) carbon atoms per molecule and can be used in internal combustion engines. Biogasoline is chemically different from biobutanol and bioethanol, as these are alcohols, not hydrocarbons.

Hydrocarbon plants are plants that follow certain metabolic pathways that produce hydrocarbon products similar to petroleum. These hydrocarbon products are called terpenoids. The plants that produce terpenoids in large enough quantities to be harvested can be as big as trees or as small as single-cell algae. The family Euphorbiaceae has been studied in detail by Dr. Melvin Calvin, Nobel Laureate, and discoverer of the Calvin Cycle. One particular tree of the genus Hevea, more commonly known as the rubber tree, is probably the most famous hydrocarbon plant, supplying an estimated one third of the world’s rubber demand. It is still not as quick and cheap to make as petroleum-based rubber, which is why it does not occupy a larger portion of the market. Hevea naturally produces a latex substance which can tapped by cutting into the tree, and the latex can then be processed into rubber.
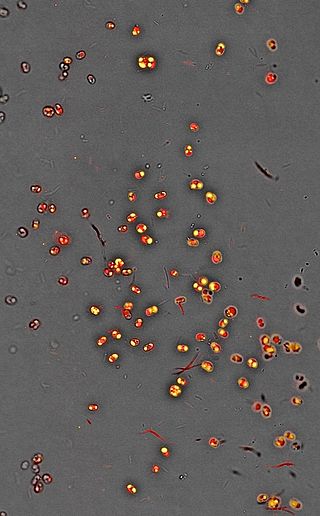
Nannochloropsis is a genus of alga within the heterokont line of eukaryotes, that is being investigated for biofuel production. One marine Nannochloropsis species has been shown to be suitable for algal biofuel production due to its ease of growth and high oil content, mainly unsaturated fatty acids and a significant percentage of palmitic acid. It also contains enough unsaturated fatty acid linolenic acid and polyunsaturated acid for a quality biodiesel.

Carbon-neutral fuel is fuel which produces no net-greenhouse gas emissions or carbon footprint. In practice, this usually means fuels that are made using carbon dioxide (CO2) as a feedstock. Proposed carbon-neutral fuels can broadly be grouped into synthetic fuels, which are made by chemically hydrogenating carbon dioxide, and biofuels, which are produced using natural CO2-consuming processes like photosynthesis.
References
- ↑ Ogi, T.; Inoue, S.; Sawayama, S.; Dote, Y. (1997). "Fuel Oil Production from Hydrocarbon-Rich Microalgae Botryococcus Braunii". In Bridgewater, A.V.; Boocock, A.V. (eds.). Developments in Thermochemical Biomass Conversion. Springer, Dordrecht. pp. 339–348. doi:10.1007/978-94-009-1559-6_27. ISBN 978-94-010-7196-3.
- ↑ Hillen, L. W.; Pollard, G.; Wake, L. V.; White, N. (January 1982). "Hydrocracking of the oils of Botryococcus braunii to transport fuels". Biotechnology and Bioengineering. 24 (1): 193–205. doi:10.1002/bit.260240116. PMID 18546110. S2CID 43310427.
- ↑ Kitazato, H.; Asaoka, S.; Iwamoto, H. (1989). "Catalytic cracking of hydrocarbons from microalgae". Journal of the Japan Petroleum Institute (in Japanese). 32 (1): 28–34. doi:10.1627/jpi1958.32.28.
- ↑ Lornabene, T.G. (1982). "Microorganisms as hydrocarbon producers". In Mislin, H.; Bachofen, R. (eds.). New Trends in Research and Utilization of Solar Energy through Biological Systems. Basel: Birkhäuser. pp. 49–52. doi:10.1007/978-3-0348-6305-6_10. ISBN 978-3-0348-6307-0.