
Tartar sauce is a condiment made of mayonnaise, chopped pickles, capers and herbs such as tarragon and dill. Tartar sauce can also be enhanced with the addition other varieties of herbs, lemon juice, or olives.
In chemistry, a racemic mixture, or racemate, is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. A sample with only a single enantiomer is an enantiomerically pure or enantiopure compound.

Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. It is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste.

Baking powder is a dry chemical leavening agent, a mixture of a carbonate or bicarbonate and a weak acid. The base and acid are prevented from reacting prematurely by the inclusion of a buffer such as cornstarch. Baking powder is used to increase the volume and lighten the texture of baked goods. It works by releasing carbon dioxide gas into a batter or dough through an acid–base reaction, causing bubbles in the wet mixture to expand and thus leavening the mixture. The first single-acting baking powder was developed by food manufacturer Alfred Bird in England in 1843. The first double-acting baking powder was developed by Eben Norton Horsford in the United States of America in the 1860s.

Potassium sodium tartrate tetrahydrate, also known as Rochelle salt, is a double salt of tartaric acid first prepared by an apothecary, Pierre Seignette, of La Rochelle, France. Potassium sodium tartrate and monopotassium phosphate were the first materials discovered to exhibit piezoelectricity. This property led to its extensive use in "crystal" gramophone (phono) pick-ups, microphones and earpieces during the post-World War II consumer electronics boom of the mid-20th Century. Such transducers had an exceptionally high output with typical pick-up cartridge outputs as much as 2 volts or more. Rochelle salt is deliquescent so any transducers based on the material deteriorated if stored in damp conditions.

Potassium bitartrate, also known as potassium hydrogen tartrate, with formula KC4H5O6, is a byproduct of winemaking. In cooking it is known as cream of tartar. It is processed from the potassium acid salt of tartaric acid (a carboxylic acid). The resulting powdery acid can be used in baking or as a cleaning solution (when mixed with an acidic solution such as lemon juice or white vinegar).
Acid salts are a class of salts that produce an acidic solution after being dissolved in a solvent. Its formation as a substance has a greater electrical conductivity than that of the pure solvent. An acidic solution formed by acid salt is made during partial neutralization of diprotic or polyprotic acids. A half-neutralization occurs due to the remaining of replaceable hydrogen atoms from the partial dissociation of weak acids that have not been reacted with hydroxide ions to create water molecules. Acid salt is an ionic compound consisted of an anion, contributed from a weak parent acid, and a cation, contributed from a strong parent base.

Dimercaptosuccinic acid (DMSA), also called succimer, is a medication used to treat lead, mercury, and arsenic poisoning. When radiolabeled with technetium-99m, it is used in a number of types of diagnostic testing. A full course is 19 days of medications by mouth. More than two weeks should pass before a second course is given.

Dimethylethanolamine (DMAE or DMEA) is an organic compound with the formula (CH3)2NCH2CH2OH. It is bifunctional, containing both a tertiary amine and primary alcohol functional groups. It is a colorless viscous liquid. It is used in skin care products. It is prepared by the ethoxylation of dimethylamine.
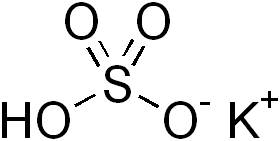
Potassium bisulfate is an inorganic compound with the chemical formula KHSO4 and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid.
In biochemical protein targeting, a peroxisomal targeting signal (PTS) is a region of the peroxisomal protein that receptors recognize and bind to. It is responsible for specifying that proteins containing this motif are localised to the peroxisome.

Cysteamine is a chemical compound that can be biosynthesized in mammals, including humans, by the degradation of coenzyme A. The intermediate pantetheine is broken down into cysteamine and pantothenic acid. It is the biosynthetic precursor to the neurotransmitter hypotaurine.

Disodium pyrophosphate or sodium acid pyrophosphate (SAPP) is an inorganic compound consisting of sodium cations and pyrophosphate anion. It is a white, water-soluble solid that serves as a buffering and chelating agent, with many applications in the food industry. When crystallized from water, it forms a hexahydrate, but it dehydrates above room temperature. Pyrophosphate is a polyvalent anion with a high affinity for polyvalent cations, e.g. Ca2+.

Chicoric acid is a hydroxycinnamic acid, an organic compound of the phenylpropanoid class and occurs in a variety of plant species. It is a derivative of both caffeic acid and tartaric acid.

Aluminium acetotartrate is an organic acid, astringent, and disinfectant. It is the aluminium salt of acetic acid and tartaric acid.
TRX2 is a dietary supplement marketed for individuals with hair loss. It is manufactured and sold by Oxford BioLabs in the United Kingdom, marketed in 2011.

Recombinant human parathyroid hormone, sold under the brand names Preotact and Natpara, is an artificially manufactured form of the parathyroid hormone (H05AA03) used to treat hypoparathyroidism. Recombinant human parathyroid hormone is used in the treatment of osteoporosis in postmenopausal women at high risk of osteoporotic fractures. A significant reduction in the incidence of vertebral fractures has been demonstrated.

Uvitic acid (5-methylisophthalic acid) is an organic compound with the formula CH3C6H3(COOH)2. The name comes from Latin uva which means a grape. The acid is called so because it may be produced indirectly from tartaric acid, which is found in the grape. Under normal conditions, the acid is a white crystalline substance.
Fruit salt or fruit salts is a term for effervescent compounds made up of organic acids such as citric acid or tartaric acid and salts such as sodium bicarbonate, sodium carbonate, or sodium bitartrate in combination with added flavoring and sugar. Historically, fruit salts were sold for a wide range of ailments, and today they are used primarily as antacids.














