
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.
Omega−3 fatty acids, also called Omega−3 oils, ω−3 fatty acids, Ω-3 Fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, being important constituents of animal lipid metabolism, and they play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ALA can be found in plants, while DHA and EPA are found in algae and fish. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. DHA and EPA accumulate in fish that eat these algae. Common sources of plant oils containing ALA include walnuts, edible seeds, and flaxseeds as well as hempseed oil, while sources of EPA and DHA include fish and fish oils, and algae oil.

Lipoic acid (LA), also known as α-lipoic acid, alpha-lipoic acid (ALA) and thioctic acid, is an organosulfur compound derived from caprylic acid (octanoic acid). ALA is made in animals normally, and is essential for aerobic metabolism. It is also manufactured and is available as a dietary supplement in some countries where it is marketed as an antioxidant, and is available as a pharmaceutical drug in other countries. Lipoate is the conjugate base of lipoic acid, and the most prevalent form of LA under physiological conditions. Only the (R)-(+)-enantiomer (RLA) exists in nature and is essential for aerobic metabolism because RLA is an essential cofactor of many enzyme complexes.

α-Linolenic acid, also known as alpha-linolenic acid (ALA), is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.
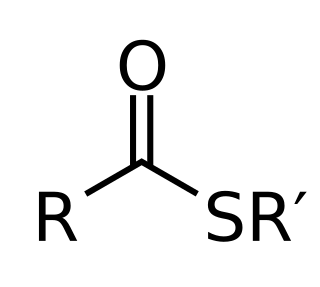
In organic chemistry, thioesters are organosulfur compounds with the molecular structure R−C(=O)−S−R’. They are analogous to carboxylate esters with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid with a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA. The R and R' represent organyl groups, or H in the case of R.
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.
Linoleic acid (LA) is an organic compound with the formula HOOC(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.

Microalgae or microphytes are microscopic algae invisible to the naked eye. They are phytoplankton typically found in freshwater and marine systems, living in both the water column and sediment. They are unicellular species which exist individually, or in chains or groups. Depending on the species, their sizes can range from a few micrometers (μm) to a few hundred micrometers. Unlike higher plants, microalgae do not have roots, stems, or leaves. They are specially adapted to an environment dominated by viscous forces.
In biochemistry and nutrition, a monounsaturated fat is a fat that contains a monounsaturated fatty acid (MUFA), a subclass of fatty acid characterized by having a double bond in the fatty acid chain with all of the remaining carbon atoms being single-bonded. By contrast, polyunsaturated fatty acids (PUFAs) have more than one double bond.

Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is a primary structural component of the human brain, cerebral cortex, skin, and retina. It is given the fatty acid notation 22:6(n-3). It can be synthesized from alpha-linolenic acid or obtained directly from maternal milk, fatty fish, fish oil, or algae oil. The consumption of DHA contributes to numerous physiological benefits, including cognition. As the primary structural component of nerve cells in the brain, the function of DHA is to support neuronal conduction and to allow optimal function of neuronal membrane proteins.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.
Rose hip seed oil is a pressed seed oil, extracted from the seeds of the wild rose bush Rosa rubiginosa in the southern Andes. Rosehip seed oil can also be extracted from Rosa canina, a wild rose species native to Europe, northwest Africa, and western Asia. The fruits of the rosehip have been used in folk medicine for a long time. Rosehips have prophylactic and therapeutic actions against the common cold, infectious diseases, gastrointestinal disorders, urinary tract diseases, and inflammatory diseases.

Docosatetraenoic acid designates any straight chain 22:4 fatty acid.

Conjugated fatty acids is jargon for polyunsaturated fatty acids containing at least one pair of conjugated double bonds. An example of a conjugated fatty acid is the rumenic acid, found in the meat and milk of ruminants. Most unsaturated fatty acids that are doubly unsaturated do not feature conjugation, e.g., linoleic acid and linoelaidic acid.

2,4 Dienoyl-CoA reductase also known as DECR1 is an enzyme which in humans is encoded by the DECR1 gene which resides on chromosome 8. This enzyme catalyzes the following reactions

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.
Furan fatty acids are a group of fatty acids that contain a furan ring. To this furan ring, an unbranched carboxylic acid and, at another position, an alkyl residue are attached. Natural furan fatty acids are mono- or di-methylated on the furan ring. Furan fatty acids can be found in a variety of plant and animal species.











