
RNA editing is a molecular process through which some cells can make discrete changes to specific nucleotide sequences within an RNA molecule after it has been generated by RNA polymerase. It occurs in all living organisms and is one of the most evolutionarily conserved properties of RNAs. RNA editing may include the insertion, deletion, and base substitution of nucleotides within the RNA molecule. RNA editing is relatively rare, with common forms of RNA processing not usually considered as editing. It can affect the activity, localization as well as stability of RNAs, and has been linked with human diseases.

Potassium voltage-gated channel subfamily A member 1 also known as Kv1.1 is a shaker related voltage-gated potassium channel that in humans is encoded by the KCNA1 gene. Isaacs syndrome is a result of an autoimmune reaction against the Kv1.1 ion channel.
Missense mRNA is a messenger RNA bearing one or more mutated codons that yield polypeptides with an amino acid sequence different from the wild-type or naturally occurring polypeptide. Missense mRNA molecules are created when template DNA strands or the mRNA strands themselves undergo a missense mutation in which a protein coding sequence is mutated and an altered amino acid sequence is coded for.

Glutamate receptor 3 is a protein that in humans is encoded by the GRIA3 gene.
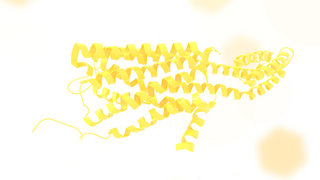
The 5-HT2C receptor is a subtype of the 5-HT2 receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, it is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. HTR2C denotes the human gene encoding for the receptor, that in humans is located on the X chromosome. As males have one copy of the gene and females have one of the two copies of the gene repressed, polymorphisms at this receptor can affect the two sexes to differing extent.

Filamin A, alpha (FLNA) is a protein that in humans is encoded by the FLNA gene.

The double-stranded RNA-specific adenosine deaminase enzyme family are encoded by the ADAR family genes. ADAR stands for adenosine deaminase acting on RNA. This article focuses on the ADAR proteins; This article details the evolutionary history, structure, function, mechanisms and importance of all proteins within this family.

Glutamate ionotropic receptor AMPA type subunit 2 is a protein that in humans is encoded by the GRIA2 gene and it is a subunit found in the AMPA receptors.

Glutamate ionotropic receptor kainate type subunit 2, also known as ionotropic glutamate receptor 6 or GluR6, is a protein that in humans is encoded by the GRIK2 gene.

Glutamate receptor, ionotropic, kainate 1, also known as GRIK1, is a protein that in humans is encoded by the GRIK1 gene.

Cytoplasmic FMR1-interacting protein 2 is a protein that in humans is encoded by the CYFIP2 gene. Cytoplasmic FMR1 interacting protein is a 1253 amino acid long protein and is highly conserved sharing 99% sequence identity to the mouse protein. It is expressed mainly in brain tissues, white blood cells and the kidney.

Glutamate receptor 4 is a protein that in humans is encoded by the GRIA4 gene.

Gamma-aminobutyric acid receptor subunit alpha-3 is a protein that in humans is encoded by the GABRA3 gene.

Uncharacterized protein KIAA1109 is a protein that in humans is encoded by the KIAA1109 gene.

Bladder cancer-associated protein is a protein that in humans is encoded by the BLCAP gene.

ADP-ribosylation-like factor 6 interacting protein 4 (ARL6IP4), also called SRp25 is the product of the ARL6IP4 gene located on chromosome 12q24. 31. Its function is unknown.

Cilia And Flagella Associated Protein 206 (CFAP206) is a gene that in humans encodes a protein “DUF3508”. This protein has a function that is not currently very well understood. Other known aliases are “dJ382I10.1, UPF0704 Protein C6orf165.” In humans, the gene coding sequence is 56,501 base pairs long, with an mRNA of 2,215 base pairs, and a protein sequence of 622 amino acids. The C6orf165 gene is conserved in chimpanzee, rhesus monkey, dog, cow, mouse, rat, chicken, zebrafish, mosquito, frog, and more C6orf165 is rarely expressed in humans, with relatively high expression in brain, lungs (trachea) and testis. The molecular weight of UPF0704 is 71,193 Da and the PI is 6.38

Uncharacterized protein C12orf60 is a protein that in humans is encoded by the C12orf60 gene. The gene is also known as LOC144608 or MGC47869. The protein lacks transmembrane domains and helices, but it is rich in alpha-helices. It is predicted to localize in the nucleus.
The split gene theory is a theory of the origin of introns, long non-coding sequences in eukaryotic genes between the exons. The theory holds that the randomness of primordial DNA sequences would only permit small (< 600bp) open reading frames (ORFs), and that important intron structures and regulatory sequences are derived from stop codons. In this introns-first framework, the spliceosomal machinery and the nucleus evolved due to the necessity to join these ORFs into larger proteins, and that intronless bacterial genes are less ancestral than the split eukaryotic genes. The theory originated with Periannan Senapathy.

GPATCH2L is a protein that is encoded by the GPATCH2L human gene located at 14q24.3. In humans, the length of mRNA in GPATCH2L (NM_017926) is 14,021 base pairs and the gene spans bases is 62,422 nt between chr14: 76,151,922 - 76,214,343. GPATCH2L is on the positive strand. IFT43 is the gene directly before GPATCH2L on the positive strand and LOC105370575 is the uncharacterized gene on the negative strand, which is approximately one and a half the size of GPATCH2L. Known aliases for GPATCH2L contain C14orf118, FLJ20689, FLJ10033, and KIAA1152. GPATCH2L produces 28 distinct introns, 17 different mRNAs, 14 alternatively spliced variants, and 3 unspliced forms. It has 5 probable alternative promoters, 7 validated polyadenylation sites, and 6 predicted promoters of varying lengths.



















