Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development.

Neuraminidase (Sialidase) enzymes are glycoside hydrolase enzymes that cleave (cut) the glycosidic linkages of neuraminic acids. Neuraminidase enzymes are a large family, found in a range of organisms. The best-known neuraminidase is the viral neuraminidase, a drug target for the prevention of the spread of influenza infection. The viral neuraminidases are frequently used as antigenic determinants found on the surface of the influenza virus. Some variants of the influenza neuraminidase confer more virulence to the virus than others. Other homologues are found in mammalian cells, which have a range of functions. At least four mammalian sialidase homologues have been described in the human genome . Sialidases may act as pathogenic factors in microbial infections.
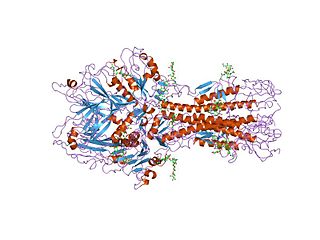
Hemagglutinin esterase (HEs) is a glycoprotein that certain enveloped viruses possess and use as invading mechanism. HEs helps in the attachment and destruction of certain sialic acid receptors that are found on the host cell surface. Viruses that possess HEs include Influenza C virus, toroviruses, and coronaviruses. HEs is a dimer transmembrane protein consisting of two monomers, each monomer is made of three domains. The three domains are: membrane fusion, esterase, and receptor binding domains.
The genome and proteins of HIV have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.

Envelope glycoprotein GP120 is a glycoprotein exposed on the surface of the HIV envelope. It was discovered by Professors Tun-Hou Lee and Myron "Max" Essex of the Harvard School of Public Health in 1988. The 120 in its name comes from its molecular weight of 120 kDa. Gp120 is essential for virus entry into cells as it plays a vital role in attachment to specific cell surface receptors. These receptors are DC-SIGN, Heparan Sulfate Proteoglycan and a specific interaction with the CD4 receptor, particularly on helper T-cells. Binding to CD4 induces the start of a cascade of conformational changes in gp120 and gp41 that lead to the fusion of the viral membrane with the host cell membrane. Binding to CD4 is mainly electrostatic although there are van der Waals interactions and hydrogen bonds.

The Matrix-2 (M2) protein is a proton-selective viroporin, integral in the viral envelope of the influenza A virus. The channel itself is a homotetramer, where the units are helices stabilized by two disulfide bonds, and is activated by low pH. The M2 protein is encoded on the seventh RNA segment together with the M1 protein. Proton conductance by the M2 protein in influenza A is essential for viral replication.
Cyanovirin-N (CV-N) is a protein produced by the cyanobacterium Nostoc ellipsosporum that displays virucidal activity against several viruses, including human immunodeficiency virus (HIV). The virucidal activity of CV-N is mediated through specific high-affinity interactions with the viral surface envelope glycoproteins gp120 and gp41, as well as to high-mannose oligosaccharides found on the HIV envelope. In addition, CV-N is active against rhinoviruses, human parainfluenza virus, respiratory syncytial virus, and enteric viruses. The virucidal activity of CV-N against influenza virus is directed towards viral haemagglutinin. CV-N has a complex fold composed of a duplication of a tandem repeat of two homologous motifs comprising three-stranded beta-sheet and beta-hairpins.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
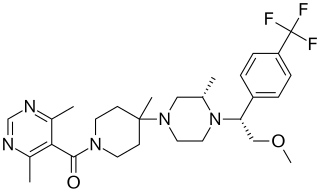
Vicriviroc, previously named SCH 417690 and SCH-D, is a pyrimidine CCR5 entry inhibitor of HIV-1. It was developed by the pharmaceutical company Schering-Plough. Merck decided to not pursue regulatory approval for use in treatment-experienced patients because the drug did not meet primary efficacy endpoints in late stage trials. Clinical trials continue in patients previously untreated for HIV.

Griffithsin is a protein isolated from the red algae Griffithsia. It has a 121-amino acid sequence which exhibits a Jacalin-like lectin fold. Several structures of this protein have been solved by X-ray crystallography and deposited in the PDB. It has been shown in vitro to be a highly potent HIV entry inhibitor. It is currently being investigated as a potential microbicide for use in the prevention of the transmission of HIV.

Mannosyl-oligosaccharide 1,2-alpha-mannosidase IA is an enzyme that in humans is encoded by the MAN1A1 gene.

GBA2 is the gene that encodes the enzyme non-lysosomal glucosylceramidase in humans. It has glucosylceramidase activity.
Many major physiological processes depend on regulation of proteolytic enzyme activity and there can be dramatic consequences when equilibrium between an enzyme and its substrates is disturbed. In this prospective, the discovery of small-molecule ligands, like protease inhibitors, that can modulate catalytic activities has an enormous therapeutic effect. Hence, inhibition of the HIV protease is one of the most important approaches for the therapeutic intervention in HIV infection and their development is regarded as major success of structure-based drug design. They are highly effective against HIV and have, since the 1990s, been a key component of anti-retroviral therapies for HIV/AIDS.
BanLec is a lectin from the jacalin-related lectin family isolated from the fruit of the bananas Musa acuminata and Musa balbisiana. BanLec is one of the predominant proteins in the pulp of ripe bananas and has binding specificity for mannose and mannose-containing oligosaccharides. A 2010 study reported that BanLec was a potent inhibitor of HIV replication.
Antiviral proteins are proteins that are induced by human or animal cells to interfere with viral replication. These proteins are isolated to inhibit the virus from replicating in a host's cells and stop it from spreading to other cells. The Pokeweed antiviral protein and the Zinc-Finger antiviral protein are two major antiviral proteins that have undergone several tests for viruses, including HIV and influenza.

Virus inhibitory protein, endoplasmic reticulum-associated, interferon-inducible (Viperin), also known as RSAD2, is a protein that is encoded by the RSAD2 gene. Viperin is a multifunctional protein in viral processes that is an interferon stimulated gene. It has been reported that viperin could be induced by either IFN-dependent or IFN-independent pathways and certain viruses may use viperin to increase their infectivity.
Scytonema varium is a cultured cyanobacterium of the genus Scytonema. It is one of many anti viral protein producing algae. In a similar manner to Cyanovirin-N from Nostoc Ellipsosporum and griffithsin from the red algae Griffithsia, Scytonema varium secretes the broad-spectrum antiviral protein scytovirin which can inactivate both the HIV virus, and Ebola virus, offering hope of treatment for many diseases with viral etiology (cause). It is currently being investigated as a topical microbicide for HIV prophylaxis.
Scytovirin is a 95-amino acid antiviral protein isolated from the cyanobacteria Scytonema varium. It has been cultured in E. coli and its structure investigated in detail. Scytovirin is thought to be produced by the bacteria to protect itself from viruses that might otherwise attack it, but as it has broad-spectrum antiviral activity against a range of enveloped viruses, scytovirin has also been found to be useful against a range of major human pathogens, most notably HIV / AIDS but also including SARS coronavirus and filoviruses such as Ebola virus and Marburg virus. While some lectins such as cyanovirin and Urtica dioica agglutinin are thought likely to be too allergenic to be used internally in humans, studies so far on scytovirin and griffithsin have not shown a similar level of immunogenicity. Scytovirin and griffithsin are currently being investigated as potential microbicides for topical use.
Ten Feizi is a British molecular biologist who is Professor and Director of the Glycosciences Laboratory at Imperial College London. Her research considers the structure and function of glycans. She was awarded the Society for Glycobiology Rosalind Kornfeld award in 2014.









