
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets and the other are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.
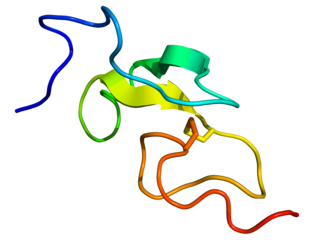
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-kDa and has 53 amino acid residues and three intramolecular disulfide bonds.

Factor IX is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia.

The epidermal growth factor receptor is a transmembrane protein that is a receptor for members of the epidermal growth factor family of extracellular protein ligands.

Transforming growth factor alpha (TGF-α) is a protein that in humans is encoded by the TGFA gene. As a member of the epidermal growth factor (EGF) family, TGF-α is a mitogenic polypeptide. The protein becomes activated when binding to receptors capable of protein kinase activity for cellular signaling.

Fibulin (FY-beau-lin) is the prototypic member of a multigene family, currently with seven members. Fibulin-1 is a calcium-binding glycoprotein. In vertebrates, fibulin-1 is found in blood and extracellular matrices. In the extracellular matrix, fibulin-1 associates with basement membranes and elastic fibers. The association with these matrix structures is mediated by its ability to interact with numerous extracellular matrix constituents including fibronectin, proteoglycans, laminins and tropoelastin. In blood, fibulin-1 binds to fibrinogen and incorporates into clots.
The ErbB family of proteins contains four receptor tyrosine kinases, structurally related to the epidermal growth factor receptor (EGFR), its first discovered member. In humans, the family includes Her1, Her2, Her3 (ErbB3), and Her4 (ErbB4). The gene symbol, ErbB, is derived from the name of a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene. Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer's disease, while excessive ErbB signaling is associated with the development of a wide variety of types of solid tumor.
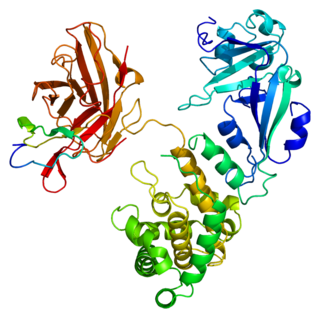
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of proteins that in humans is encoded by the HBEGF gene.

Epiregulin (EPR) is a protein that in humans is encoded by the EREG gene.
The EGF module-containing Mucin-like hormone Receptors (EMRs) are closely related subgroup of G protein-coupled receptors (GPCRs). These receptors have a unique hybrid structure in which an extracellular epidermal growth factor (EGF)-like domain is fused to a GPCR domain through a mucin-like stalk. There are four variants of EMR labeled 1-4, each encoded by a separate gene. These receptors are predominantly expressed in cells of the immune system and bind ligands such as CD55.

EGF-like module-containing mucin-like hormone receptor-like 3 is a protein encoded by the ADGRE3 gene. EMR3 is a member of the adhesion GPCR family. Adhesion GPCRs are characterized by an extended extracellular region often possessing N-terminal protein modules that is linked to a TM7 region via a domain known as the GPCR-Autoproteolysis INducing (GAIN) domain.

Wall-associated kinases (WAKs) are one of many classes of plant proteins known to serve as a medium between the extracellular matrix (ECM) and cytoplasm of cell walls. They are serine-threonine kinases that contain epidermal growth factor (EGF) repeats, a cytoplasmic kinase and are located in the cell walls. They provide a linkage between the inner and outer surroundings of cell walls. WAKs are under a group of receptor-like kinases (RLK) that are actively involved in sensory and signal transduction pathways especially in response to foreign attacks by pathogens and in cell development. On the other hand, pectins are an abundant group of complex carbohydrates present in the primary cell wall that play roles in cell growth and development, protection, plant structure and water holding capacity.
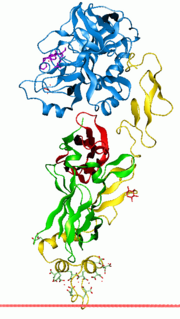
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions.

Receptor tyrosine-protein kinase erbB-3, also known as HER3, is a membrane bound protein that in humans is encoded by the ERBB3 gene.

The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.
YWTD repeats are four-stranded beta-propeller repeats found in low-density lipoprotein receptors (LDLR). The six YWTD repeats together fold into a six-bladed beta-propeller. Each blade of the propeller consists of four antiparallel beta-strands; the innermost strand of each blade is labeled 1 and the outermost strand, 4. The sequence repeats are offset with respect to the blades of the propeller, such that any given 40-residue YWTD repeat spans strands 24 of one propeller blade and strand 1 of the subsequent blade. This offset ensures circularization of the propeller because the last strand of the final sequence repeat acts as an innermost strand 1 of the blade that harbors strands 24 from the first sequence repeat. The repeat is found in a variety of proteins that include, vitellogenin receptor from Drosophila melanogaster, low-density lipoprotein (LDL) receptor, preproepidermal growth factor, and nidogen (entactin).
Fibronectin, type I repeats are one of the three repeats found in the fibronectin protein. Fibronectin is a plasma protein that binds cell surfaces and various compounds including collagen, fibrin, heparin, DNA, and actin. Type I domain (FN1) is approximately 40 residues in length. Four conserved cysteines are involved in disulfide bonds. The 3D structure of the FN1 domain has been determined. It consists of two antiparallel beta-sheets, first a double-stranded one, that is linked by a disulfide bond to a triple-stranded beta-sheet. The second conserved disulfide bridge links the C-terminal adjacent strands of the domain.
SNED1 is an extracellular matrix (ECM) protein expressed at low levels in a wide range of tissues. The gene encoding SNED1 is located in the human chromosome 2 at locus q37.3. The corresponding mRNA isolated from the spleen and is 6834bp in length, and the corresponding protein is 1413 amino-acid long. The mouse ortholog of SNED1 was cloned in 2004 from the embryonic kidney by Leimester et al. SNED1 present domains characteristic of ECM proteins, including an amino-terminal NIDO domain, several calcium binding EGF-like domains (EGF_CA), a Sushi domain also known as complement control protein (CCP) domain, and three type III fibronectin (FN3) domains in the carboxy-terminal region.

Transmembrane protein 8A is a protein that in humans is encoded by the TMEM8A gene (16p13.3.). Evolutionarily, TMEM8A orthologs are found in primates and mammals and in a few more distantly related species. TMEM8A contains five transmembrane domains and one EGF-like domain which are all highly conserved in the ortholog space. Although there is no confirmed function of TMEM8A, through analyzing expression and experimental data, it is predicted that TMEM8A is an adhesion protein that plays a role in keeping T-cells in their resting state.
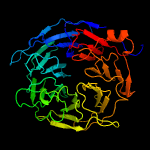
Megf8 also known as Multiple Epidermal Growth Factor-like Domains 8, is a protein coding gene that encodes a single pass membrane protein, known to participate in developmental regulation and cellular communication. It is located on chromosome 19 at the 49th open reading frame in humans (19q13.2). There are two isoform constructs known for MEGF8, which differ by a 67 amino acid indel. The isoform 2 splice version is 2785 amino acids long, and predicted to be 296.6 kdal in mass. Isoform 1 is composed of 2845 amino acids and predicted to weigh 303.1 kdal. Using BLAST searches, orthologs were found primarily in mammals, but MEGF8 is also conserved in invertebrates and fishes, and rarely in birds, reptiles, and amphibians. A notably important paralog to multiple epidermal growth factor-like domains 8 is ATRNL1, which is also a single pass transmembrane protein, with several of the same key features and motifs as MEGF8, as indicated by Simple Modular Architecture Research Tool (SMART) which is hosted by the European Molecular Biology Laboratory located in Heidelberg, Germany. MEGF8 has been predicted to be a key player in several developmental processes, such as left-right patterning and limb formation. Currently, researchers have found MEGF8 SNP mutations to be the cause of Carpenter syndrome subtype 2.















