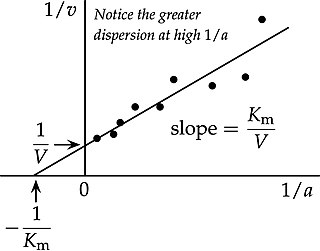
In biochemistry, the Lineweaver–Burk plot is a graphical representation of the Michaelis–Menten equation of enzyme kinetics, described by Hans Lineweaver and Dean Burk in 1934.

Aminolevulinic acid synthase (ALA synthase, ALAS, or delta-aminolevulinic acid synthase) is an enzyme (EC 2.3.1.37) that catalyzes the synthesis of δ-aminolevulinic acid (ALA) the first common precursor in the biosynthesis of all tetrapyrroles such as hemes, cobalamins and chlorophylls. The reaction is as follows:

In biochemistry, ABTS is a chemical compound used to observe the reaction kinetics of specific enzymes. A common use for it is in the enzyme-linked immunosorbent assay (ELISA) to detect the binding of molecules to each other.

Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier might affect the rate.
Microbial collagenase is an enzyme. This enzyme catalyses the following chemical reaction

Phosphoglycerate mutase (PGM) is any enzyme that catalyzes step 8 of glycolysis - the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG) through a 2,3-bisphosphoglycerate intermediate. These enzymes are categorized into the two distinct classes of either cofactor-dependent (dPGM) or cofactor-independent (iPGM). The dPGM enzyme is composed of approximately 250 amino acids and is found in all vertebrates as well as in some invertebrates, fungi, and bacteria. The iPGM class is found in all plants and algae as well as in some invertebrate, fungi, and Gram-positive bacteria. This class of PGM enzyme shares the same superfamily as alkaline phosphatase.

William Wallace Cleland (January 6, 1930 – March 6, 2013, often cited as W. W. Cleland, and known almost universally as "Mo Cleland", was a University of Wisconsin-Madison biochemistry professor. His research was concerned with enzyme reaction mechanism and enzyme kinetics, especially multiple-substrate enzymes. He was elected to the National Academy of Sciences in 1985.

Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme (EC 2.1.2.1) which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate (THF) to 5,10-Methylenetetrahydrofolate (5,10-CH2-THF). This reaction provides the largest part of the one-carbon units available to the cell.

Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes is the deamination of L-serine to yield pyruvate, with the release of ammonia.
In enzymology, a ferredoxin—nitrate reductase (EC 1.7.7.2) is an enzyme that catalyzes the chemical reaction

In enzymology, a methylenetetrahydrofolate dehydrogenase (NADP+) (EC 1.5.1.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a cysteine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-glycerol diphosphatase (EC 3.6.1.16) is an enzyme that catalyzes the chemical reaction
In enzymology, a diiodotyrosine transaminase is an enzyme that catalyzes the chemical reaction
In enzymology, a polyphosphate-glucose phosphotransferase is an enzyme that catalyzes the chemical reaction.
In the field of enzymology, a proton ATPase is an enzyme that catalyzes the following chemical reaction:
Biochimica et Biophysica Acta (BBA) is a peer-reviewed scientific journal in the field of biochemistry and biophysics that was established in 1947. The journal is published by Elsevier with a total of 100 annual issues in ten specialised sections.

Dipeptidyl peptidase I is an enzyme. This enzyme catalyses the following chemical reaction
o-Aminophenol oxidase (EC 1.10.3.4, isophenoxazine synthase, o-aminophenol:O2 oxidoreductase, 2-aminophenol:O2 oxidoreductase, GriF) is an enzyme with systematic name 2-aminophenol:oxygen oxidoreductase. This enzyme catalyses the following chemical reaction
NADH dehydrogenase is an enzyme that converts nicotinamide adenine dinucleotide (NAD) from its reduced form (NADH) to its oxidized form (NAD+). Members of the NADH dehydrogenase family and analogues are commonly systematically named using the format NADH:acceptor oxidoreductase. The chemical reaction these enzymes catalyze is generally represented with the following equation:









