A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Indigo is a term used for a number of hues in the region of blue. The word comes from the ancient dye of the same name. The term "indigo" can refer to the color of the dye, various colors of fabric dyed with indigo dye, a spectral color, one of the seven colors of the rainbow, or a region on the color wheel, and can include various shades of blue, ultramarine, and green-blue. Since the web era, the term has also been used for various purple and violet hues identified as "indigo", based on use of the term "indigo" in HTML web page specifications.

Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical study.

Indigo dye is an organic compound with a distinctive blue color. Indigo is a natural dye extracted from the leaves of some plants of the Indigofera genus, in particular Indigofera tinctoria; dye-bearing Indigofera plants were commonly grown and used throughout the world, in Asia in particular, as an important crop, with the production of indigo dyestuff economically important due to the historical rarity of other blue dyestuffs.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.
A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

Dyeing is the application of dyes or pigments on textile materials such as fibers, yarns, and fabrics with the goal of achieving color with desired color fastness. Dyeing is normally done in a special solution containing dyes and particular chemical material. Dye molecules are fixed to the fiber by absorption, diffusion, or bonding with temperature and time being key controlling factors. The bond between the dye molecule and fiber may be strong or weak, depending on the dye used. Dyeing and printing are different applications; in printing, color is applied to a localized area with desired patterns. In dyeing, it is applied to the entire textile.
Indanthrone blue, also called indanthrene, is an organic compound with the formula (C14H6O2NH)2. It is a dark blue solid that is a common dye as well as a precursor to other dyes.
Vat dyes are a class of dyes that are classified as such because of the method by which they are applied. Vat dyeing is a process that refers to dyeing that takes place in a bucket or vat. The original vat dye is indigo, once obtained only from plants but now often produced synthetically.

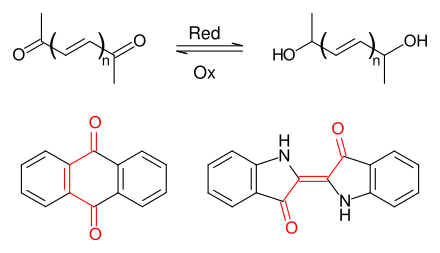
A leuco dye is a dye which can switch between two chemical forms, one of which is colorless. Reversible transformations can be caused by heat, light or pH, resulting in examples of thermochromism, photochromism and halochromism respectively. Irreversible transformations typically involve reduction or oxidation. The colorless form is sometimes referred to as the leuco form.

Quinizarine Green SS, also called Solvent Green 3 is an anthraquinone derivative. It is a black powder that is soluble in polar organic solvents, but insoluble in water. It is used as a dye for adding greenish coloring to cosmetics and medications. It is used in some colored smoke formulations.

For the parent molecule 9,10-anthraquinone, see anthraquinone

Henry Edward Schunck, also known as Edward von Schunck, was a British chemist who did much work with dyes.

Kerria lacca is a species of insect in the family Kerriidae, the lac insects. These are in the superfamily Coccoidea, the scale insects. This species is perhaps the most commercially important lac insect, being a main source of lac, a resin which can be refined into shellac and other products. This insect is native to Asia.
A dihydroxyanthraquinone is any of several isomeric organic compounds with formula C
14H
8O
4, formally derived from 9,10-anthraquinone by replacing two hydrogen atoms by hydroxyl groups. Dihyroxyantraquinones have been studied since the early 1900s, and include some compounds of historical and current importance. The isomers differ in the position of the hydroxyl groups, and of the carbonyl oxygens (=O) of the underlying anthraquinone.

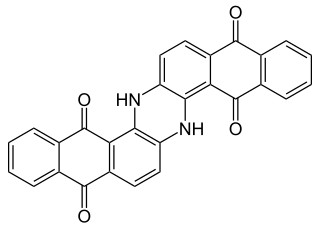
Anthraquinone dyes are an abundant group of dyes comprising a anthraquinone unit as the shared structural element. Anthraquinone itself is colourless, but red to blue dyes are obtained by introducing electron donor groups such as hydroxy or amino groups in the 1-, 4-, 5- or 8-position. Anthraquinone dyestuffs are structurally related to indigo dyestuffs and are classified together with these in the group of carbonyl dyes.

Natural dyes are dyes or colorants derived from plants, invertebrates, or minerals. The majority of natural dyes are vegetable dyes from plant sources—roots, berries, bark, leaves, and wood—and other biological sources such as fungi.
Disperse dye is a category of synthetic dye intended for polyester and related hydrophobic fibers. Disperse dyes are polar molecules containing anthraquinone or azo groups. It is estimated that 85% of disperse dyes are azos or anthraquinone dyes.
The Bohn–Schmidt reaction, a named reaction in chemistry, introduces a hydroxy group at an anthraquinone system. The anthraquinone must already have at least one hydroxy group. The reaction was first described in 1889 by René Bohn (1862–1922) and in 1891 by Robert Emanuel Schmidt (1864–1938), two German industrial chemists.