Eukaryotic
Eukaryotic cells [2] are cells that consist of membrane-bound organelles, a membrane-bound nucleus, and more than one linear chromosome. Being much more complex than prokaryotic cells, cells without a true nucleus, eukaryotes must protect its organelles from outside forces.

Plant
Plant cell mechanics combines principles of biomechanics and mechanobiology to investigate the growth and shaping of the plant cells. Plant cells, similar to animal cells, respond to externally applied forces, such as by reorganization of their cytoskeletal network. The presence of a considerably rigid extracellular matrix, the cell wall, however, bestows the plant cells with a set of particular properties. Mainly, the growth of plant cells is controlled by the mechanics and chemical composition of the cell wall. [3] A major part of research in plant cell mechanics is put toward the measurement and modeling of the cell wall mechanics to understand how modification of its composition and mechanical properties affects the cell function, growth and morphogenesis. [4] [5]
Animal
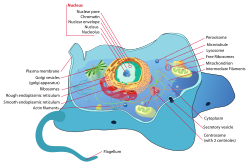
Because animal cells [6] do not have cell walls to protect them like plant cells, they require other specialized structures to sustain external mechanical forces. All animal cells are encased within a cell membrane made of a thin lipid bilayer that protects the cell from exposure to the outside environment. Using receptors composed of protein structures, the cell membrane is able to let selected molecules within the cell. Inside the cell membrane includes the cytoplasm, which contains the cytoskeleton. [7] A network of filamentous proteins including microtubules, intermediate filaments, and actin filaments makes up the cytoskeleton and helps maintain the cell's shape. By working together, the three types of polymers can organize themselves to counter the applied external forces and resist deformation. However, there are differences between the three polymers.
The primary structural component of the cytoskeleton is actin filaments. Being the narrowest with a diameter of 7 nm and most flexible out of the three types of polymers, actin filaments are typically found at the very edge of the cytoplasm in animal cells. [1] Formed by the linking of polymers of a protein called actin, they help give cells shape and structure and are able to transport protein packages and organelles. Furthermore, actin filaments have the ability to be assembled and disassembled quickly, allowing them to take part in cell mobility. [8]
On the other hand, intermediate filaments are more permanent structures with a diameter of 8 to 10 nm. [9] Composed of numerous fibrous protein strands wound together, intermediate proteins' main role is bearing tension and retaining the shape and structure of the cell by securing the nucleus and other organelles in their designated areas.
The largest cytoskeletal structure of the three types of polymers is the microtubules with a diameter of 25 nm. [8] Unlike actin filaments, microtubules are stiff, hollow structures that radiate outwards from the microtubule organizing center (MTOC). Composed of tubulin proteins, microtubules are dynamic structures that allows them to shrink or grow with the addition or removal of tubulin proteins. In terms of cell mechanics, microtubules' main purpose is to resist compressive cellular forces and act as a transportation system for motor proteins. [8]
It was shown that melanin also can have an impact on mechanic properties of cells. The research done by Sarna's team proved that heavily pigmented melanoma cells have Young's modulus about 4.93, when in non-pigmented ones it was only 0.98. [10] In another experiment they found that elasticity of melanoma cells is important for its metastasis and growth: non-pigmented tumors were bigger than pigmented and it was much easier for them to spread. They shown that there are both pigmented and non-pigmented cells in melanoma tumors, so that they can both be drug-resistant and metastatic. [10]