Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. Progesterone has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.

A steroid is an organic compound with four fused rings arranged in a specific molecular configuration.

Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.

A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids and sex steroids. Within those two classes are five types according to the receptors to which they bind: glucocorticoids and mineralocorticoids and androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands.
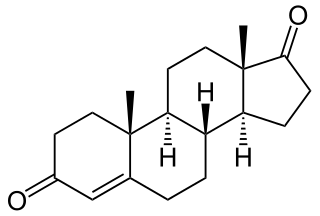
Androstenedione, or 4-androstenedione, also known as androst-4-ene-3,17-dione, is an endogenous weak androgen steroid hormone and intermediate in the biosynthesis of estrone and of testosterone from dehydroepiandrosterone (DHEA). It is closely related to androstenediol (androst-5-ene-3β,17β-diol).
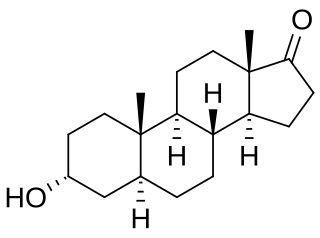
Androsterone, or 3α-hydroxy-5α-androstan-17-one, is an endogenous steroid hormone, neurosteroid, and putative pheromone. It is a weak androgen with a potency that is approximately 1/7 that of testosterone. Androsterone is a metabolite of testosterone and dihydrotestosterone (DHT). In addition, it can be converted back into DHT via 3α-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase, bypassing conventional intermediates such as androstanedione and testosterone, and as such, can be considered to be a metabolic intermediate in its own right.
Saponins, also selectively referred to as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed but found particularly in soapwort, a flowering plant, the soapbark tree and soybeans. They are used in soaps, medicines, fire extinguishers, speciously as dietary supplements, for synthesis of steroids, and in carbonated beverages. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.

Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.

Pterobranchia, members of which are often called pterobranchs, is a class of small worm-shaped animals. They belong to the Hemichordata, and live in secreted tubes on the ocean floor. Pterobranchia feed by filtering plankton out of the water with the help of cilia attached to tentacles. There are about 25 known living pterobranch species in three genera, which are Rhabdopleura, Cephalodiscus, and Atubaria. On the other hand, there are several hundred extinct genera, some of which date from the Cambrian Period.

The Fujimoto–Belleau reaction is a chemical reaction that forms cyclic α-substituted α,β-unsaturated ketones from enol lactones. The reaction was discovered in 1951 by George I. Fujimoto and Bernard Belleau. Belleau used this reaction to synthesize 1-methyl-3-keto-1,2,3,9,10,10a-hexahydrophenanthrene from a ketoacid starting material and Fujimoto demonstrated that steroids could be synthesized from naturally occurring lactone species using this method as well.
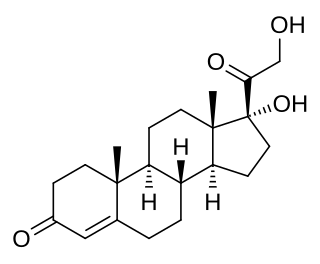
11-Deoxycortisol, also known as cortodoxone (INN), cortexolone as well as 17α,21-dihydroxyprogesterone or 17α,21-dihydroxypregn-4-ene-3,20-dione, is an endogenous glucocorticoid steroid hormone, and a metabolic intermediate toward cortisol. It was first described by Tadeusz Reichstein in 1938 as Substance S, thus has also been referred to as Reichstein's Substance S or Compound S.
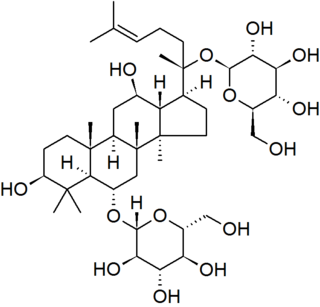
Ginsenosides or panaxosides are a class of natural product steroid glycosides and triterpene saponins. Compounds in this family are found almost exclusively in the plant genus Panax (ginseng), which has a long history of use in traditional medicine that has led to the study of pharmacological effects of ginseng compounds. As a class, ginsenosides exhibit a large variety of subtle and difficult-to-characterize biological effects when studied in isolation.

Arenobufagin is a cardiotoxic bufanolide steroid secreted by the Argentine toad Bufo arenarum. It has effects similar to digitalis, blocking the Na+/K+ pump in heart tissue.
Abraliopsis gilchristi is a species of enoploteuthid cephalopods found in southern temperate waters of the south Pacific Ocean, from New Zealand to South Africa, where it is abundant. It undergoes a vertical daily migration, spending the day at depth and moving closer to the surface at night to feed on copepods, euphausiids and hyperiids. Spawning appears to occur between September and December. The specific name honours the Scottish zoologist John Gilchrist (1866-1926) who was the first director of the Marine Biological Survey in Cape Town. The type specimen was taken off Cape Town and is held in the Natural History Museum, London.

Cephalodiscus is a genus of hemichordates in the monotypic family Cephalodiscidae of the order Cephalodiscida.

7β-Hydroxydehydroepiandrosterone, also known as 3β,7β-dihydroxyandrost-5-ene-17-one, is an endogenous, naturally occurring steroid and a metabolite of dehydroepiandrosterone (DHEA). The major metabolic pathway of DHEA outside the liver is via 7-hydroxylation into 7α-OH-DHEA and 7β-OH-DHEA. 7β-OH-DHEA has weak antiestrogenic activity, selectively antagonizing the estrogen receptor ERβ.
Cephalodiscus densus is a sessile hemichordate belonging to the order Cephalodiscida. The species is endemic to the Antarctic, being found in the Ross Sea and much of the Antarctic coastline, as well as near the Kerguelen Islands.

Cephalodiscus nigrescens is a sessile hemichordate belonging to the order Cephalodiscida.
Cephalodiscus planitectus is a sessile hemichordate belonging to the order Cephalodiscida.