A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range (nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
A biosensor is an analytical device, used for the detection of a chemical substance, that combines a biological component with a physicochemical detector. The sensitive biological element, e.g. tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc., is a biologically derived material or biomimetic component that interacts with, binds with, or recognizes the analyte under study. The biologically sensitive elements can also be created by biological engineering. The transducer or the detector element, which transforms one signal into another one, works in a physicochemical way: optical, piezoelectric, electrochemical, electrochemiluminescence etc., resulting from the interaction of the analyte with the biological element, to easily measure and quantify. The biosensor reader device connects with the associated electronics or signal processors that are primarily responsible for the display of the results in a user-friendly way. This sometimes accounts for the most expensive part of the sensor device, however it is possible to generate a user friendly display that includes transducer and sensitive element. The readers are usually custom-designed and manufactured to suit the different working principles of biosensors.
Nanosensors are nanoscale devices that measure physical quantities and convert these to signals that can be detected and analyzed. There are several ways proposed today to make nanosensors; these include top-down lithography, bottom-up assembly, and molecular self-assembly. There are different types of nanosensors in the market and in development for various applications, most notably in defense, environmental, and healthcare industries. These sensors share the same basic workflow: a selective binding of an analyte, signal generation from the interaction of the nanosensor with the bio-element, and processing of the signal into useful metrics.

James Mitchell Tour is an American chemist and nanotechnologist. He is a Professor of Chemistry, Professor of Materials Science and Nanoengineering at Rice University in Houston, Texas.

Nanocomposite is a multiphase solid material where one of the phases has one, two or three dimensions of less than 100 nanometers (nm) or structures having nano-scale repeat distances between the different phases that make up the material.

Carbon nanotubes (CNTs) are cylinders of one or more layers of graphene (lattice). Diameters of single-walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes (MWNTs) are typically 0.8 to 2 nm and 5 to 20 nm, respectively, although MWNT diameters can exceed 100 nm. CNT lengths range from less than 100 nm to 0.5 m.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.
Magnetic nanoparticles (MNPs) are a class of nanoparticle that can be manipulated using magnetic fields. Such particles commonly consist of two components, a magnetic material, often iron, nickel and cobalt, and a chemical component that has functionality. While nanoparticles are smaller than 1 micrometer in diameter, the larger microbeads are 0.5–500 micrometer in diameter. Magnetic nanoparticle clusters that are composed of a number of individual magnetic nanoparticles are known as magnetic nanobeads with a diameter of 50–200 nanometers. Magnetic nanoparticle clusters are a basis for their further magnetic assembly into magnetic nanochains. The magnetic nanoparticles have been the focus of much research recently because they possess attractive properties which could see potential use in catalysis including nanomaterial-based catalysts, biomedicine and tissue specific targeting, magnetically tunable colloidal photonic crystals, microfluidics, magnetic resonance imaging, magnetic particle imaging, data storage, environmental remediation, nanofluids, optical filters, defect sensor, magnetic cooling and cation sensors.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.

Transparent conducting films (TCFs) are thin films of optically transparent and electrically conductive material. They are an important component in a number of electronic devices including liquid-crystal displays, OLEDs, touchscreens and photovoltaics. While indium tin oxide (ITO) is the most widely used, alternatives include wider-spectrum transparent conductive oxides (TCOs), conductive polymers, metal grids and random metallic networks, carbon nanotubes (CNT), graphene, nanowire meshes and ultra thin metal films.
Nanoarchitectures for lithium-ion batteries are attempts to employ nanotechnology to improve the design of lithium-ion batteries. Research in lithium-ion batteries focuses on improving energy density, power density, safety, durability and cost.

Carbon nanotube chemistry involves chemical reactions, which are used to modify the properties of carbon nanotubes (CNTs). CNTs can be functionalized to attain desired properties that can be used in a wide variety of applications. The two main methods of CNT functionalization are covalent and non-covalent modifications.
Carbide-derived carbon (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as MAX phases (e.g., Ti2AlC, Ti3SiC2). CDCs have also been derived from polymer-derived ceramics such as Si-O-C or Ti-C, and carbonitrides, such as Si-N-C. CDCs can occur in various structures, ranging from amorphous to crystalline carbon, from sp2- to sp3-bonded, and from highly porous to fully dense. Among others, the following carbon structures have been derived from carbide precursors: micro- and mesoporous carbon, amorphous carbon, carbon nanotubes, onion-like carbon, nanocrystalline diamond, graphene, and graphite. Among carbon materials, microporous CDCs exhibit some of the highest reported specific surface areas (up to more than 3000 m2/g). By varying the type of the precursor and the CDC synthesis conditions, microporous and mesoporous structures with controllable average pore size and pore size distributions can be produced. Depending on the precursor and the synthesis conditions, the average pore size control can be applied at sub-Angstrom accuracy. This ability to precisely tune the size and shapes of pores makes CDCs attractive for selective sorption and storage of liquids and gases (e.g., hydrogen, methane, CO2) and the high electric conductivity and electrochemical stability allows these structures to be effectively implemented in electrical energy storage and capacitive water desalinization.

Single-walled carbon nanohorn is the name given by Sumio Iijima and colleagues in 1999 to horn-shaped sheath aggregate of graphene sheets. Very similar structures had been observed in 1994 by Peter J.F. Harris, Edman Tsang, John Claridge and Malcolm Green. Ever since the discovery of the fullerene, the family of carbon nanostructures has been steadily expanded. Included in this family are single-walled and multi-walled carbon nanotubes, carbon onions and cones and, most recently, SWNHs. These SWNHs with about 40–50 nm in tubule length and about 2–3 nm in diameter are derived from SWNTs and ended by a five-pentagon conical cap with a cone opening angle of ~20o. Moreover, thousands of SWNHs associate with each other to form the ‘dahlia-like' and ‘bud-like’ structured aggregates which have an average diameter of about 80–100 nm. The former consists of tubules and graphene sheets protruding from its surface like petals of a dahlia, while the latter is composed of tubules developing inside the particle itself. Their unique structures with high surface area and microporosity make SWNHs become a promising material for gas adsorption, biosensing, drug delivery, gas storage and catalyst support for fuel cell. Single-walled carbon nanohorns are an example of the family of carbon nanocones.
Potential graphene applications include lightweight, thin, and flexible electric/photonics circuits, solar cells, and various medical, chemical and industrial processes enhanced or enabled by the use of new graphene materials, and favoured by massive cost decreases in graphene production.

In materials and electric battery research, cobalt oxide nanoparticles usually refers to particles of cobalt(II,III) oxide Co
3O
4 of nanometer size, with various shapes and crystal structures.
In materials science, vertically aligned carbon nanotube arrays (VANTAs) are a unique microstructure consisting of carbon nanotubes oriented with their longitudinal axis perpendicular to a substrate surface. These VANTAs effectively preserve and often accentuate the unique anisotropic properties of individual carbon nanotubes and possess a morphology that may be precisely controlled. VANTAs are consequently widely useful in a range of current and potential device applications.
Niveen M. Khashab is a Lebanese chemist and an associate Professor of chemical Sciences and engineering at King Abdullah University of Science and Technology in Saudi Arabia since 2009. She is a laureate of the 2017 L'Oréal-UNESCO Awards for Women in Science "for her contributions to innovative smart hybrid materials aimed at drug delivery and for developing new techniques to monitor intracellular antioxidant activity." She is also a fellow of the Royal Chemical Society, and a member of the American Chemical Society.

Graphenated carbon nanotubes (G-CNTs) are a relatively new hybrid that combines graphitic foliates grown along the sidewalls of multiwalled or bamboo style carbon nanotubes (CNTs). Yu et al. reported on "chemically bonded graphene leaves" growing along the sidewalls of CNTs. Stoner et al. described these structures as "graphenated CNTs" and reported in their use for enhanced supercapacitor performance. Hsu et al. further reported on similar structures formed on carbon fiber paper, also for use in supercapacitor applications. Pham et al. also reported a similar structure, namely "graphene-carbon nanotube hybrids", grown directly onto carbon fiber paper to form an integrated, binder free, high surface area conductive catalyst support for Proton Exchange Membrane Fuel Cells electrode applications with enhanced performance and durability. The foliate density can vary as a function of deposition conditions with their structure ranging from few layers of graphene to thicker, more graphite-like.
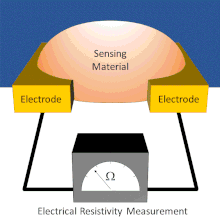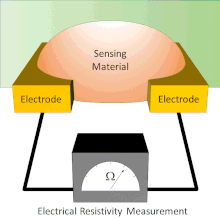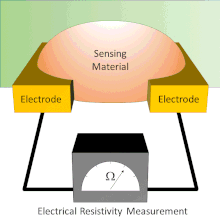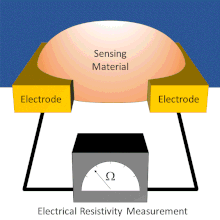
A chemical sensor array is a sensor architecture with multiple sensor components that create a pattern for analyte detection from the additive responses of individual sensor components. There exist several types of chemical sensor arrays including electronic, optical, acoustic wave, and potentiometric devices. These chemical sensor arrays can employ multiple sensor types that are cross-reactive or tuned to sense specific analytes.