
In cell biology a centriole is a cylindrical organelle composed mainly of a protein called tubulin. Centrioles are found in most eukaryotic cells, but are not present in conifers (Pinophyta), flowering plants (angiosperms) and most fungi, and are only present in the male gametes of charophytes, bryophytes, seedless vascular plants, cycads, and Ginkgo. A bound pair of centrioles, surrounded by a highly ordered mass of dense material, called the pericentriolar material (PCM), makes up a structure called a centrosome.

Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

A flagellum is a hairlike appendage that protrudes from certain plant and animal sperm cells, from fungal spores (zoospores), and from a wide range of microorganisms to provide motility. Many protists with flagella are known as flagellates.

The cilium is a membrane-bound organelle found on most types of eukaryotic cell. Cilia are absent in bacteria and archaea. The cilium has the shape of a slender threadlike projection that extends from the surface of the much larger cell body. Eukaryotic flagella found on sperm cells and many protozoans have a similar structure to motile cilia that enables swimming through liquids; they are longer than cilia and have a different undulating motion.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.

Cytokinesis is the part of the cell division process and part of mitosis during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and meiosis. During cytokinesis the spindle apparatus partitions and transports duplicated chromatids into the cytoplasm of the separating daughter cells. It thereby ensures that chromosome number and complement are maintained from one generation to the next and that, except in special cases, the daughter cells will be functional copies of the parent cell. After the completion of the telophase and cytokinesis, each daughter cell enters the interphase of the cell cycle.
The microtubule-organizing center (MTOC) is a structure found in eukaryotic cells from which microtubules emerge. MTOCs have two main functions: the organization of eukaryotic flagella and cilia and the organization of the mitotic and meiotic spindle apparatus, which separate the chromosomes during cell division. The MTOC is a major site of microtubule nucleation and can be visualized in cells by immunohistochemical detection of γ-tubulin. The morphological characteristics of MTOCs vary between the different phyla and kingdoms. In animals, the two most important types of MTOCs are 1) the basal bodies associated with cilia and flagella and 2) the centrosome associated with spindle formation.

A basal body is a protein structure found at the base of a eukaryotic undulipodium. The basal body was named by Theodor Wilhelm Engelmann in 1880. It is formed from a centriole and several additional protein structures, and is, essentially, a modified centriole. The basal body serves as a nucleation site for the growth of the axoneme microtubules. Centrioles, from which basal bodies are derived, act as anchoring sites for proteins that in turn anchor microtubules, and are known as the microtubule organizing center (MTOC). These microtubules provide structure and facilitate movement of vesicles and organelles within many eukaryotic cells.

In molecular biology, an axoneme, also called an axial filament, is the microtubule-based cytoskeletal structure that forms the core of a cilium or flagellum. Cilia and flagella are found on many cells, organisms, and microorganisms, to provide motility. The axoneme serves as the "skeleton" of these organelles, both giving support to the structure and, in some cases, the ability to bend. Though distinctions of function and length may be made between cilia and flagella, the internal structure of the axoneme is common to both.

Stathmin, also known as metablastin and oncoprotein 18 is a protein that in humans is encoded by the STMN1 gene.
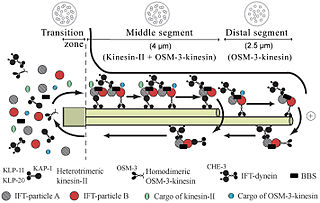
Intraflagellar transport (IFT) is a bidirectional motility along axoneme microtubules that is essential for the formation (ciliogenesis) and maintenance of most eukaryotic cilia and flagella. It is thought to be required to build all cilia that assemble within a membrane projection from the cell surface. Plasmodium falciparum cilia and the sperm flagella of Drosophila are examples of cilia that assemble in the cytoplasm and do not require IFT. The process of IFT involves movement of large protein complexes called IFT particles or trains from the cell body to the ciliary tip and followed by their return to the cell body. The outward or anterograde movement is powered by kinesin-2 while the inward or retrograde movement is powered by cytoplasmic dynein 2/1b. The IFT particles are composed of about 20 proteins organized in two subcomplexes called complex A and B.

Centrosomal protein of 290 kDa is a protein that in humans is encoded by the CEP290 gene. CEP290 is located on the Q arm of chromosome 12.

A ciliopathy is any genetic disorder that affects the cellular cilia or the cilia anchoring structures, the basal bodies, or ciliary function. Primary cilia are important in guiding the process of development, so abnormal ciliary function while an embryo is developing can lead to a set of malformations that can occur regardless of the particular genetic problem. The similarity of the clinical features of these developmental disorders means that they form a recognizable cluster of syndromes, loosely attributed to abnormal ciliary function and hence called ciliopathies. Regardless of the actual genetic cause, it is clustering of a set of characteristic physiological features which define whether a syndrome is a ciliopathy.
Mechanosensation is the transduction of mechanical stimuli into neural signals. Mechanosensation provides the basis for the senses of light touch, hearing, proprioception, and pain. Mechanoreceptors found in the skin, called cutaneous mechanoreceptors, are responsible for the sense of touch. Tiny cells in the inner ear, called hair cells, are responsible for hearing and balance. States of neuropathic pain, such as hyperalgesia and allodynia, are also directly related to mechanosensation. A wide array of elements are involved in the process of mechanosensation, many of which are still not fully understood.
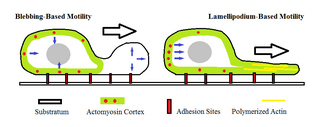
Amoeboid movement is the most typical mode of locomotion in adherent eukaryotic cells. It is a crawling-like type of movement accomplished by protrusion of cytoplasm of the cell involving the formation of pseudopodia ("false-feet") and posterior uropods. One or more pseudopodia may be produced at a time depending on the organism, but all amoeboid movement is characterized by the movement of organisms with an amorphous form that possess no set motility structures.
A BBSome is a protein complex that operates in primary cilia biogenesis, homeostasis, and intraflagellar transport (IFT). The BBSome recognizes cargo proteins and signaling molecules like G-protein coupled receptors (GPCRs) on the ciliary membrane and helps transport them to and from the primary cilia. Primary cilia are nonmotile microtubule projections that function like antennae and are found in many types of cells. They receive various environmental signals to aid the cell in survival. They can detect photons by concentrating rhodopsin, a light receptor that converts photons into chemical signals, or odorants by concentrating olfactory receptors on the primary cilia surface. Primary cilia are also meaningful in cell development and signaling. They do not contain any way to make proteins within the primary cilia, so the BBSome aids in transporting essential proteins to, from, and within the cilia. Examples of cargo proteins that the BBSome is responsible for ferrying include smoothened, polycystic-1 (PC1), and several G-Protein coupled receptors (GPCRs) like somatostatin receptors (Sstr3), melanin-concentrating hormone receptor 1 (Mchr1), and neuropeptide Y2 receptor.
Cytosolic ciliogenesis, otherwise cytoplasmic ciliogenesis, is a type of ciliogenesis where the cilium axoneme is formed in the cytoplasm or becomes exposed to the cytoplasm.
Compartmentalized ciliogenesis is the most common type of ciliogenesis where the cilium axoneme is formed separated from the cytoplasm by the ciliary membrane and a ciliary gate known as the transition zone.
RVxP motif is a protein motif involved in localizing proteins into cilia.

Jeremy Reiter is an American developmental geneticist who is the chair of the Department of Biochemistry and Biophysics at the University of California, San Francisco (UCSF). He is holder of the Albert Bowers Endowed Chair. His research focuses on the cilium, particularly in understanding its role in cell signaling and its involvement in human diseases such as cancer, congenital disorders and obesity.














