
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen, via the fragment antigen-binding (Fab) variable region. Each tip of the "Y" of an antibody contains a paratope that is specific for one particular epitope on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize its target directly. Depending on the antigen, the binding may impede the biological process causing the disease or may activate macrophages to destroy the foreign substance. The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region, which contains a conserved glycosylation site involved in these interactions. The production of antibodies is the main function of the humoral immune system.

B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system by secreting antibodies. Additionally, B cells present antigens and secrete cytokines. In mammals, B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick, and not from bone marrow as commonly believed.
Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells, acting at around 3 days after infection, and respond to tumor formation. Typically, immune cells detect the major histocompatibility complex (MHC) presented on infected cell surfaces, triggering cytokine release, causing lysis or apoptosis. NK cells are unique, however, as they have the ability to recognize stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the initial notion that they do not require activation to kill cells that are missing "self" markers of MHC class 1. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.

Monoclonal antibodies are antibodies that are made by identical immune cells that are all clones of a unique parent cell. Monoclonal antibodies can have monovalent affinity, in that they bind to the same epitope. In contrast, polyclonal antibodies bind to multiple epitopes and are usually made by several different plasma cell lineages. Bispecific monoclonal antibodies can also be engineered, by increasing the therapeutic targets of one single monoclonal antibody to two epitopes.

Hybridoma technology is a method for producing large numbers of identical antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal B cell cancer cells, a myeloma, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma. The hybridomas can be grown in culture, each culture starting with one viable hybridoma cell, producing cultures each of which consists of genetically identical hybridomas which produce one antibody per culture (monoclonal) rather than mixtures of different antibodies (polyclonal). The myeloma cell line that is used in this process is selected for its ability to grow in tissue culture and for an absence of antibody synthesis. In contrast to polyclonal antibodies, which are mixtures of many different antibody molecules, the monoclonal antibodies produced by each hybridoma line are all chemically identical.
Chimeric antigen receptor T cells are T cells that have been genetically engineered to produce an artificial T-cell receptor for use in immunotherapy.

Cancer immunotherapy is the artificial stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspeciality of oncology.

The adaptive immune system, also referred as the acquired immune system, is a subsystem of the immune system that is composed of specialized, systemic cells and processes that eliminates pathogens by preventing their growth. The acquired immune system is one of the two main immunity strategies found in vertebrates.

Clonal selection theory is a scientific theory in immunology that explains the functions of cells of the immune system (lymphocytes) in response to specific antigens invading the body. The concept was introduced by Australian doctor Frank Macfarlane Burnet in 1957, in an attempt to explain the great diversity of antibodies formed during initiation of the immune response. The theory has become the widely accepted model for how the human immune system responds to infection and how certain types of B and T lymphocytes are selected for destruction of specific antigens.

Germinal centers or germinal centres (GCs) are sites within secondary lymphoid organs – lymph nodes and the spleen where mature B cells proliferate, differentiate, and mutate their antibody genes during a normal immune response to an infection. These develop dynamically after the activation of follicular B cells by T-dependent antigen.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAb) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. More recently antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
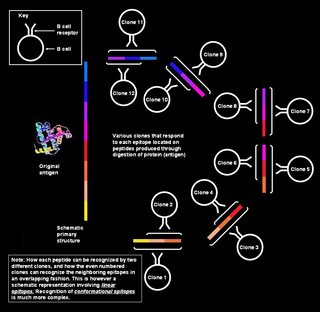
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts, called epitopes, by multiple clones of B cell.
Small modular immunopharmaceuticals, or SMIPs for short, are artificial proteins that are intended for use as pharmaceutical drugs. They are largely built from parts of antibodies (immunoglobulins), and like them have a binding site for antigens that could be used for monoclonal antibody therapy. SMIPs have similar biological half-life and, being smaller than antibodies, are reasoned to have better tissue penetration properties. They were invented by Trubion and are now being developed by Emergent BioSolutions, which acquired Trubion in 2010.

B-cell prolymphocytic leukemia, referred to as B-PLL, is a rare blood cancer. It is a more aggressive, but still treatable, form of leukemia.
Plasma cell dyscrasias are a spectrum of progressively more severe monoclonal gammopathies in which a clone or multiple clones of pre-malignant or malignant plasma cells over-produce and secrete into the blood stream a myeloma protein, i.e. an abnormal monoclonal antibody or portion thereof. The exception to this rule is the disorder termed non-secretory multiple myeloma; this disorder is a form of plasma cell dyscrasia in which no myeloma protein is detected in serum or urine of individuals who have clear evidence of an increase in clonal bone marrow plasma cells and/or evidence of clonal plasma cell-mediated tissue injury. Here, a clone of plasma cells refers to group of plasma cells that are abnormal in that they have an identical genetic identity and therefore are descendants of a single genetically distinct ancestor cell.

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.

Two chemically linked fragments antigen-binding form an artificial antibody that binds to two different antigens, making it a type of bispecific antibody. They are fragments antigen-binding of two different monoclonal antibodies and are linked by chemical means like a thioether. Typically, one of the Fabs binds to a tumour antigen and the other to a protein on the surface of an immune cell, for example an Fc receptor on a macrophage. In this way, tumour cells are attached to immune cells, which destroy them.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
NK-92 is an immortal cell line that has features and characteristics of natural killer (NK) cells that every person has circulating in the blood. Blood NK cells and NK-92 cells recognize invaders such as viruses and fungi. NK-92 cells, like blood NK cells, can attack cancer cells if the tumor has not grown out of control. NK-92 cells were isolated and characterized by the laboratory of Hans Klingemann at the British Columbia Cancer Agency in Vancouver, Canada. The cells came from a patient who had a NK cell lymphoma, a rare lymphoma type. Although several other NK cell lines have been cloned, only NK-92 cells can be expanded to larger numbers and consistently kill tumor cells. When NK-92 cells bind to a cancer or infected cell, they secrete perforin, which punches holes in target cells, followed by granzymes, which induce apoptosis in the target cells. NK-92 cells also attack cancer cells through the Fas-Fas ligand system and are capable of producing cytokines that by themselves can kill cancer cells or stimulate and expand other immune cells such as interferon.
Complement-dependent cytotoxicity (CDC) is an effector function of IgG and IgM antibodies. When they are bound to surface antigen on target cell, the classical complement pathway is triggered by bonding protein C1q to these antibodies, resulting in formation of a membrane attack complex (MAC) and target cell lysis.