In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that cause disease. Antibodies can recognize virtually any size antigen, able to perceive diverse chemical compositions. Each antibody recognizes one or more specific antigens. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each tip of the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as cancer cells, parasitic worms, and also objects such as wood splinters, distinguishing them from the organism's own healthy tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use molecules and cells to perform their functions.

Immunology is a branch of biology and medicine that covers the study of immune systems in all organisms.

In immunology, autoimmunity is the system of immune responses of an organism against its own healthy cells, tissues and other normal body constituents. Any disease resulting from this type of immune response is termed an "autoimmune disease". Prominent examples include celiac disease, diabetes mellitus type 1, Henoch–Schönlein purpura, systemic lupus erythematosus, Sjögren syndrome, eosinophilic granulomatosis with polyangiitis, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, Addison's disease, rheumatoid arthritis, ankylosing spondylitis, polymyositis, dermatomyositis, and multiple sclerosis. Autoimmune diseases are very often treated with steroids.

B cells, also known as B lymphocytes, are a type of white blood cell of the lymphocyte subtype. They function in the humoral immunity component of the adaptive immune system. B cells produce antibody molecules which may be either secreted or inserted into the plasma membrane where they serve as a part of B-cell receptors. When a naïve or memory B cell is activated by an antigen, it proliferates and differentiates into an antibody-secreting effector cell, known as a plasmablast or plasma cell. In addition, B cells present antigens and secrete cytokines. In mammals B cells mature in the bone marrow, which is at the core of most bones. In birds, B cells mature in the bursa of Fabricius, a lymphoid organ where they were first discovered by Chang and Glick, which is why the B stands for bursa and not bone marrow, as commonly believed.

A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens such as viruses or bacteria, or cells that are damaged in other ways.

Sir Frank Macfarlane Burnet, usually known as Macfarlane or Mac Burnet, was an Australian virologist known for his contributions to immunology. He won a Nobel Prize in 1960 for predicting acquired immune tolerance. He also developed the theory of clonal selection.

Niels Kaj Jerne, FRS was a Danish immunologist. He shared the Nobel Prize in Physiology or Medicine in 1984 with Georges J. F. Köhler and César Milstein "for theories concerning the specificity in development and control of the immune system and the discovery of the principle for production of monoclonal antibodies".

The adaptive immune system, AIS, also known as the acquired immune system, or specific immune system is a subsystem of the immune system that is composed of specialized cells, organs, and processes that eliminate pathogens specifically. The acquired immune system is one of the two main immunity strategies found in vertebrates.

The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.
In immunology, central tolerance is the process of eliminating any developing T or B lymphocytes that are autoreactive, i.e. reactive to the body itself. Through elimination of autoreactive lymphocytes, tolerance ensures that the immune system does not attack self peptides. Lymphocyte maturation occurs in primary lymphoid organs such as the bone marrow and the thymus. In mammals, B cells mature in the bone marrow and T cells mature in the thymus.
Memory T cells are a subset of T lymphocytes that might have some of the same functions as memory B cells. Their lineage is unclear.
Immune tolerance, also known as immunological tolerance or immunotolerance, refers to the immune system's state of unresponsiveness to substances or tissues that would otherwise trigger an immune response. It arises from prior exposure to a specific antigen and contrasts the immune system's conventional role in eliminating foreign antigens. Depending on the site of induction, tolerance is categorized as either central tolerance, occurring in the thymus and bone marrow, or peripheral tolerance, taking place in other tissues and lymph nodes. Although the mechanisms establishing central and peripheral tolerance differ, their outcomes are analogous, ensuring immune system modulation.
The following are notable events in the Timeline of immunology:
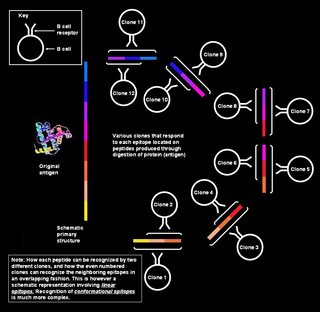
Polyclonal B cell response is a natural mode of immune response exhibited by the adaptive immune system of mammals. It ensures that a single antigen is recognized and attacked through its overlapping parts, called epitopes, by multiple clones of B cell.
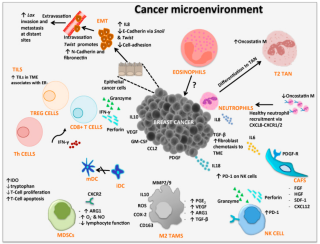
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.
In immunology, clonal deletion is the process of removing T and B lymphocytes from the immune system repertoire. The process of clonal deletion helps prevent recognition and destruction of the self host cells, making it a type of negative selection. Ultimately, clonal deletion plays a role in central tolerance. Clonal deletion can help protect individuals against autoimmunity, which is when an organism produces and immune response on its own cells. It is one of many methods used by the body in immune tolerance.
The immune network theory is a theory of how the adaptive immune system works, that has been developed since 1974 mainly by Niels Jerne and Geoffrey W. Hoffmann. The theory states that the immune system is an interacting network of lymphocytes and molecules that have variable (V) regions. These V regions bind not only to things that are foreign to the vertebrate, but also to other V regions within the system. The immune system is therefore seen as a network, with the components connected to each other by V-V interactions.

The danger model of the immune system proposes that it differentiates between components that are capable of causing damage, rather than distinguishing between self and non-self.