
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host-pathogen interface.

Porins are beta barrel proteins that cross a cellular membrane and act as a pore, through which molecules can diffuse. Unlike other membrane transport proteins, porins are large enough to allow passive diffusion, i.e., they act as channels that are specific to different types of molecules. They are present in the outer membrane of gram-negative bacteria and some gram-positive mycobacteria, the outer membrane of mitochondria, and the outer chloroplast membrane.
Virulence factors are cellular structures, molecules and regulatory systems that enable microbial pathogens to achieve the following:
Adenylate cyclase toxin is a virulence factor produced by some members of the genus Bordetella. Together with the pertussis toxin it is the most important virulence factor of the causative agent of whooping cough, Bordetella pertussis. Bordetella bronchiseptica and Bordetella parapertussis, also able to cause pertussis-like symptoms, also produce adenylate cyclase toxin. It is a toxin secreted by the bacteria to influence the host immune system.
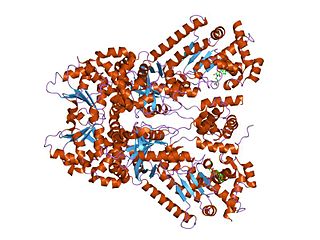
Anthrax toxin is a three-protein exotoxin secreted by virulent strains of the bacterium, Bacillus anthracis—the causative agent of anthrax. The toxin was first discovered by Harry Smith in 1954. Anthrax toxin is composed of a cell-binding protein, known as protective antigen (PA), and two enzyme components, called edema factor (EF) and lethal factor (LF). These three protein components act together to impart their physiological effects. Assembled complexes containing the toxin components are endocytosed. In the endosome, the enzymatic components of the toxin translocate into the cytoplasm of a target cell. Once in the cytosol, the enzymatic components of the toxin disrupts various immune cell functions, namely cellular signaling and cell migration. The toxin may even induce cell lysis, as is observed for macrophage cells. Anthrax toxin allows the bacteria to evade the immune system, proliferate, and ultimately kill the host animal. Research on anthrax toxin also provides insight into the generation of macromolecular assemblies, and on protein translocation, pore formation, endocytosis, and other biochemical processes.
Tetanolysin is a toxin produced by Clostridium tetani bacteria. Its function is unknown, but it is believed to contribute to the pathogenesis of tetanus. The other C. tetani toxin, tetanospasmin, is more definitively linked to tetanus. It is sensitive to oxygen.

A colicin is a type of bacteriocin produced by and toxic to some strains of Escherichia coli. Colicins are released into the environment to reduce competition from other bacterial strains. Colicins bind to outer membrane receptors, using them to translocate to the cytoplasm or cytoplasmic membrane, where they exert their cytotoxic effect, including depolarisation of the cytoplasmic membrane, DNase activity, RNase activity, or inhibition of murein synthesis.

Pore-forming proteins are usually produced by bacteria, and include a number of protein exotoxins but may also be produced by other organisms such as earthworms, who produce lysenin. They are frequently cytotoxic, as they create unregulated pores in the membrane of targeted cells.

Hemolysins or haemolysins are lipids and proteins that cause lysis of red blood cells by disrupting the cell membrane. Although the lytic activity of some microbe-derived hemolysins on red blood cells may be of great importance for nutrient acquisition, many hemolysins produced by pathogens do not cause significant destruction of red blood cells during infection. However, hemolysins are often capable of lysing red blood cells in vitro.
Listeriolysin O (LLO) is a hemolysin produced by the bacterium Listeria monocytogenes, the pathogen responsible for causing listeriosis. The toxin may be considered a virulence factor, since it is crucial for the virulence of L. monocytogenes.
The Membrane Attack Complex/Perforin (MACPF) superfamily, sometimes referred to as the MACPF/CDC superfamily, is named after a domain that is common to the membrane attack complex (MAC) proteins of the complement system and perforin (PF). Members of this protein family are pore-forming toxins (PFTs). In eukaryotes, MACPF proteins play a role in immunity and development.

Alpha-toxin, also known as alpha-hemolysin (Hla), is the major cytotoxic agent released by bacterium Staphylococcus aureus and the first identified member of the pore forming beta-barrel toxin family. This toxin consists mostly of beta-sheets (68%) with only about 10% alpha-helices. The hly gene on the S. aureus chromosome encodes the 293 residue protein monomer, which forms heptameric units on the cellular membrane to form a complete beta-barrel pore. This structure allows the toxin to perform its major function, development of pores in the cellular membrane, eventually causing cell death.
Streptolysins are two hemolytic exotoxins from Streptococcus. Types include streptolysin O, which is oxygen-labile, and streptolysin S, which is oxygen-stable.
Clostridium enterotoxins are toxins produced by Clostridium species.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxin. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.
The RTX toxin superfamily is a group of cytolysins and cytotoxins produced by bacteria. There are over 1000 known members with a variety of functions. The RTX family is defined by two common features: characteristic repeats in the toxin protein sequences, and extracellular secretion by the type I secretion systems (T1SS). The name RTX refers to the glycine and aspartate-rich repeats located at the C-terminus of the toxin proteins, which facilitate export by a dedicated T1SS encoded within the rtx operon.
The thiol-activated Cholesterol-dependent Cytolysin(CDC) family is a member of the MACPF superfamily. Cholesterol dependent cytolysins are a family of β-barrel pore-forming exotoxins that are secreted by gram-positive bacteria. CDCs are secreted as water-soluble monomers of 50-70 kDa, that when bound to the target cell, form a circular homo-oligomeric complex containing as many as 40 monomers. Through multiple conformational changes, the β-barrel transmembrane structure is formed and inserted into the target cell membrane. The presence of cholesterol in the target membrane is required for pore formation, though the presence of cholesterol is not required by all CDCs for binding. For example, intermedilysin secreted by Streptococcus intermedius will bind only to target membranes containing a specific protein receptor, independent of the presence of cholesterol, but cholesterol is required by intermedilysin for pore formation. While the lipid environment of cholesterol in the membrane can affect toxin binding, the exact molecular mechanism that cholesterol regulates the cytolytic activity of the CDC is not fully understood.
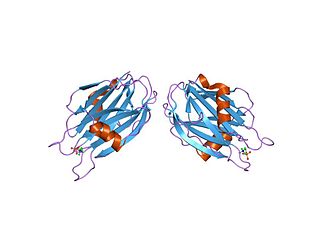
In molecular biology, the sea anemone cytotoxic proteins are lethal pore-forming proteins, known collectively as actinoporins, a sub-class of cytolysins. There are several different groups of cytolysins based on their structure and function. This entry represents the most numerous group, the 20kDa highly basic peptides. These cytolysins form cation-selective pores in sphingomyelin-containing membranes. Examples include equinatoxins, sticholysins, magnificalysins, and tenebrosins, which exhibit pore-forming, haemolytic, cytotoxic, and heart stimulatory activities.
Nanosponges are a type of nanoparticle, often a synthesized carbon-containing polymer. They are porous in structure, pores being about 1–2 nanometers in size, and can therefore be targeted to absorb small amounts of matter or toxin. Nanosponges are often used in medicine as targeted drug delivery systems, detoxification methods, or as a way of damage control after an injury. They can also be used in environmental applications to clean up ecosystems by performing tasks like purifying water or metal deposits. Their small size allows them to move quickly through substances, like water or blood, efficiently finding and attacking unwanted matter. Nanosponges are often synthetically manufactured but oftentimes include natural materials to improve their efficiency when injected into the body. Nanosponges are superior to microsponges in application as the smaller size allows less disruption into the system in which it is implemented therefore imposing less risk of failed or detrimental effects. The prefix "nano" implies that items of this size are measured on a scale of meters.
Lysenin is a pore-forming toxin (PFT) present in the coelomic fluid of the earthworm Eisenia fetida. Pore-forming toxins are a group of proteins that act as virulence factors of several pathogenic bacteria. Lysenin proteins are chiefly involved in the defense against eukaryotic and prokaryotic pathogens. Following the general mechanism of action of PFTs lysenin is segregated as a soluble monomer that binds specifically to a membrane receptor, sphingomyelin in the case of lysenin. After attaching to the membrane, the oligomerization begins, resulting in a nonamer on top of membrane, known as a prepore. After a conformational change, which could be triggered by a decrease of pH, the oligomer is inserted into the membrane in the so-called pore state.











