
Kyanite is a typically blue aluminosilicate mineral, found in aluminium-rich metamorphic pegmatites and sedimentary rock. It is the high pressure polymorph of andalusite and sillimanite, and the presence of kyanite in metamorphic rocks generally indicates metamorphism deep in the Earth's crust. Kyanite is also known as disthene or cyanite.

Magma is the molten or semi-molten natural material from which all igneous rocks are formed. Magma is found beneath the surface of the Earth, and evidence of magmatism has also been discovered on other terrestrial planets and some natural satellites. Besides molten rock, magma may also contain suspended crystals and gas bubbles.

Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the plagioclase (sodium-calcium) feldspars and the alkali (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust, and 41% of the Earth's continental crust by weight.

Metamorphic rocks arise from the transformation of existing rock to new types of rock in a process called metamorphism. The original rock (protolith) is subjected to temperatures greater than 150 to 200 °C and, often, elevated pressure of 100 megapascals (1,000 bar) or more, causing profound physical or chemical changes. During this process, the rock remains mostly in the solid state, but gradually recrystallizes to a new texture or mineral composition. The protolith may be an igneous, sedimentary, or existing metamorphic rock.

Plagioclase is a series of tectosilicate (framework silicate) minerals within the feldspar group. Rather than referring to a particular mineral with a specific chemical composition, plagioclase is a continuous solid solution series, more properly known as the plagioclase feldspar series. This was first shown by the German mineralogist Johann Friedrich Christian Hessel (1796–1872) in 1826. The series ranges from albite to anorthite endmembers (with respective compositions NaAlSi3O8 to CaAl2Si2O8), where sodium and calcium atoms can substitute for each other in the mineral's crystal lattice structure. Plagioclase in hand samples is often identified by its polysynthetic crystal twinning or 'record-groove' effect.

Metamorphism is the transformation of existing rock to rock with a different mineral composition or texture. Metamorphism takes place at temperatures in excess of 150 to 200 °C, and often also at elevated pressure or in the presence of chemically active fluids, but the rock remains mostly solid during the transformation. Metamorphism is distinct from weathering or diagenesis, which are changes that take place at or just beneath Earth's surface.

Andesite is a volcanic rock of intermediate composition. In a general sense, it is the intermediate type between silica-poor basalt and silica-rich rhyolite. It is fine-grained (aphanitic) to porphyritic in texture, and is composed predominantly of sodium-rich plagioclase plus pyroxene or hornblende.

Petrology is the branch of geology that studies rocks and the conditions under which they form. Petrology has three subdivisions: igneous, metamorphic, and sedimentary petrology. Igneous and metamorphic petrology are commonly taught together because they both contain heavy use of chemistry, chemical methods, and phase diagrams. Sedimentary petrology is, on the other hand, commonly taught together with stratigraphy because it deals with the processes that form sedimentary rock.

The lithology of a rock unit is a description of its physical characteristics visible at outcrop, in hand or core samples, or with low magnification microscopy. Physical characteristics include colour, texture, grain size, and composition. Lithology may refer to either a detailed description of these characteristics, or a summary of the gross physical character of a rock. Examples of lithologies in the second sense include sandstone, slate, basalt, or limestone.

Peridotite ( PERR-ih-doh-tyte, pə-RID-ə-) is a dense, coarse-grained igneous rock consisting mostly of the silicate minerals olivine and pyroxene. Peridotite is ultramafic, as the rock contains less than 45% silica. It is high in magnesium (Mg2+), reflecting the high proportions of magnesium-rich olivine, with appreciable iron. Peridotite is derived from Earth's mantle, either as solid blocks and fragments, or as crystals accumulated from magmas that formed in the mantle. The compositions of peridotites from these layered igneous complexes vary widely, reflecting the relative proportions of pyroxenes, chromite, plagioclase, and amphibole.

Hornfels is the group name for a set of contact metamorphic rocks that have been baked and hardened by the heat of intrusive igneous masses and have been rendered massive, hard, splintery, and in some cases exceedingly tough and durable. These properties are due to fine grained non-aligned crystals with platy or prismatic habits, characteristic of metamorphism at high temperature but without accompanying deformation. The term is derived from the German word Hornfels, meaning "hornstone", because of its exceptional toughness and texture both reminiscent of animal horns. These rocks were referred to by miners in northern England as whetstones.

The chlorites are the group of phyllosilicate minerals common in low-grade metamorphic rocks and in altered igneous rocks. Greenschist, formed by metamorphism of basalt or other low-silica volcanic rock, typically contains significant amounts of chlorite.

Cumulate rocks are igneous rocks formed by the accumulation of crystals from a magma either by settling or floating. Cumulate rocks are named according to their texture; cumulate texture is diagnostic of the conditions of formation of this group of igneous rocks. Cumulates can be deposited on top of other older cumulates of different composition and colour, typically giving the cumulate rock a layered or banded appearance.
The tholeiitic magma series is one of two main magma series in subalkaline igneous rocks, the other being the calc-alkaline series. A magma series is a chemically distinct range of magma compositions that describes the evolution of a mafic magma into a more evolved, silica rich end member. Rock types of the tholeiitic magma series include tholeiitic basalt, ferro-basalt, tholeiitic basaltic andesite, tholeiitic andesite, dacite and rhyolite. The variety of basalt in the series was originally called tholeiite but the International Union of Geological Sciences recommends that tholeiitic basalt be used in preference to that term.

Fractional crystallization, or crystal fractionation, is one of the most important geochemical and physical processes operating within crust and mantle of a rocky planetary body, such as the Earth. It is important in the formation of igneous rocks because it is one of the main processes of magmatic differentiation. Fractional crystallization is also important in the formation of sedimentary evaporite rocks.

In geology, an igneous intrusion is a body of intrusive igneous rock that forms by crystallization of magma slowly cooling below the surface of the Earth. Intrusions have a wide variety of forms and compositions, illustrated by examples like the Palisades Sill of New York and New Jersey; the Henry Mountains of Utah; the Bushveld Igneous Complex of South Africa; Shiprock in New Mexico; the Ardnamurchan intrusion in Scotland; and the Sierra Nevada Batholith of California.
An isograd is a concept used in the study of metamorphic rocks. The metamorphic grade of such a rock is a rough measure of the degree of metamorphism it has undergone, as characterised by the presence of certain index minerals. An isograd is a theoretical surface composed of points where the metamorphic grade is the same. It thus separates metamorphic zones whose rocks contain different index minerals.
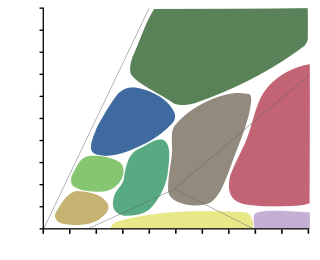
A metamorphic facies is a set of mineral assemblages in metamorphic rocks formed under similar pressures and temperatures. The assemblage is typical of what is formed in conditions corresponding to an area on the two dimensional graph of temperature vs. pressure. Rocks which contain certain minerals can therefore be linked to certain tectonic settings, times and places in the geological history of the area. The boundaries between facies are wide because they are gradational and approximate. The area on the graph corresponding to rock formation at the lowest values of temperature and pressure is the range of formation of sedimentary rocks, as opposed to metamorphic rocks, in a process called diagenesis.
Igneous rock, or magmatic rock, is one of the three main rock types, the others being sedimentary and metamorphic. Igneous rock is formed through the cooling and solidification of magma or lava.

A petrogenetic grid is a geological phase diagram that connects the stability ranges or metastability ranges of metamorphic minerals or mineral assemblages to the conditions of metamorphism. Experimentally determined mineral or mineral-assemblage stability ranges are plotted as metamorphic reaction boundaries in a pressure–temperature cartesian coordinate system to produce a petrogenetic grid for a particular rock composition. The regions of overlap of the stability fields of minerals form equilibrium mineral assemblages used to determine the pressure–temperature conditions of metamorphism. This is particularly useful in geothermobarometry.




















