The soybean, soy bean, or soya bean is a species of legume native to East Asia, widely grown for its edible bean, which has numerous uses.
The globulins are a family of globular proteins that have higher molecular weights than albumins and are insoluble in pure water but dissolve in dilute salt solutions. Some globulins are produced in the liver, while others are made by the immune system. Globulins, albumins, and fibrinogen are the major blood proteins. The normal concentration of globulins in human blood is about 2.6-3.5 g/dL.

Gliadin is a class of proteins present in wheat and several other cereals within the grass genus Triticum. Gliadins, which are a component of gluten, are essential for giving bread the ability to rise properly during baking. Gliadins and glutenins are the two main components of the gluten fraction of the wheat seed. This gluten is found in products such as wheat flour. Gluten is split about evenly between the gliadins and glutenins, although there are variations found in different sources.
Aleurone is a protein found in protein granules of maturing seeds and tubers. The term also describes one of the two major cell types of the endosperm, the aleurone layer. The aleurone layer is the outermost layer of the endosperm, followed by the inner starchy endosperm. This layer of cells is sometimes referred to as the peripheral endosperm. It lies between the pericarp and the hyaline layer of the endosperm. Unlike the cells of the starchy endosperm, aleurone cells remain alive at maturity. The ploidy of the aleurone is (3n) [as a result of double fertilization].
Hordein is a prolamin glycoprotein, present in barley and some other cereals, together with gliadin and other glycoproteins coming under the general name of gluten. Hordeins are found in the endosperm where one of their functions is to act as a storage unit.
Prolamins are a group of plant storage proteins having a high proline amino acid content. They are found in plants, mainly in the seeds of cereal grains such as wheat (gliadin), barley (hordein), rye (secalin), corn (zein), sorghum (kafirin), and oats (avenin). They are characterised by a high glutamine and proline content, and have poor solubility in water. They solubilise best in strong alcohol (70–80%), light acid, and alkaline solutions. The prolamins of the tribe Triticeae, such as wheat gliadin, and related proteins are known to trigger coeliac disease, an autoimmune condition, in genetically predisposed individuals.

Wheat allergy is an allergy to wheat which typically presents itself as a food allergy, but can also be a contact allergy resulting from occupational exposure. Like all allergies, wheat allergy involves immunoglobulin E and mast cell response. Typically the allergy is limited to the seed storage proteins of wheat. Some reactions are restricted to wheat proteins, while others can react across many varieties of seeds and other plant tissues. Wheat allergy is rare. Prevalence in adults was found to be 0.21% in a 2012 study in Japan.

Soy protein is a protein that is isolated from soybean. It is made from soybean meal that has been dehulled and defatted. Dehulled and defatted soybeans are processed into three kinds of high protein commercial products: soy flour, concentrates, and isolates. Soy protein isolate has been used since 1959 in foods for its functional properties.

Gluten is the seed storage protein in mature wheat seeds. It is the sticky substance in bread wheat which allows dough to rise and retain its shape during baking. The same, or very similar, proteins are also found in related grasses within the tribe Triticeae. Seed glutens of some non-Triticeae plants have similar properties, but none can perform on a par with those of the Triticeae taxa, particularly the Triticum species. What distinguishes bread wheat from these other grass seeds is the quantity of these proteins and the level of subcomponents, with bread wheat having the highest protein content and a complex mixture of proteins derived from three grass species.
Glutelins are a class of prolamin proteins found in the endosperm of certain seeds of the grass family. They constitute a major component of the protein composite collectively referred to as gluten. Glutenin is the most common glutelin, as it is found in wheat and is responsible for some of the refined baking properties in bread wheat. The glutelins of barley and rye have also been identified. Glutelins are the primary protein form of energy storage in the endosperm of rice grains.
Storage proteins serve as biological reserves of metal ions and amino acids, used by organisms. They are found in plant seeds, egg whites, and milk.
Edestin, is a highly-digestible, hexameric legumin protein with six subunits, and a seed storage protein, with a molecular weight of 310 kDa. This protein is primarily found in hemp seeds. Edestin is a globular protein as opposed to fibrous protein (structural).
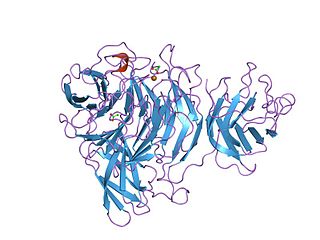
The Kelch motif is a region of protein sequence found widely in proteins from bacteria and eukaryotes. This sequence motif is composed of about 50 amino acid residues which form a structure of a four stranded beta-sheet "blade". This sequence motif is found in between five and eight tandem copies per protein which fold together to form a larger circular solenoid structure called a beta-propeller domain.
Legumin is family of globular proteins obtained from beans, peas, lentils, vetches, hemp and other leguminous seeds. Garden peas are a common nutritional source for humans that contains legumin.

Pea protein is a food product and protein supplement derived and extracted from yellow and green split peas, Pisum sativum. It can be used as a dietary supplement to increase an individual's protein or other nutrient intake, or as a substitute for other food products. As a powder, it is used as an ingredient in food manufacturing, such as a thickener, foaming agent, or an emulsifier.

In molecular biology, the YjeF N terminal is a protein domain found in the N-terminal of the protein, EDC3. The YjeF N-terminal domains occur either as single proteins or fusions with other domains and are commonly associated with enzymes. They help assemble the processing body (P-body) in preparation for mRNAdecay. Structural homology indicated it may have some similarity to the enzyme family, hydrolase.
Canavalin is a plant protein found in the jack bean, sword bean, and related plants. It is the major storage protein found in these plants' seeds, and is one of four proteins readily isolated from the seeds; the others are concanavalin A, concanavalin B, and urease. Canavalin is a vicilin protein homologous to phaseolin.
Vicilin is a legumin-associated globulin protein. It is a storage protein found in legumes such as the pea or lentil that protects plants from fungi and microorganism. It is believed to be an allergen in pea and peanut allergy responses.
11S globulin family is a family of globulin proteins chiefly found in seeds of legumes (legumin-like), along with 7S family, often found in a protein fraction within a protein isolate. They are used as storage of important nutrients for plant growth, and therefore hardy enough to pass through the human digestive system unscathed. One common example of an 11S globulin includes glycinin derived from soy.
Cruciferin is one of the two most abundant seed storage proteins in mustard and rapeseed. They are classified as 11S globulins based on their sedimentation coefficient, and are salt soluble neutral glycoproteins. Their molecular weights range from 20 to 40 kDa. They comprise up to 50–70% of the total seed protein. Cruciferin is a comparatively larger seed storage protein than napin. It is composed of two polypeptide chains α and β. The α-chain has a mass of 30 kDa and the β-chain weighs in at 20 kDa. They are held together by a disulphide bond.