
Cephems are a sub-group of β-lactam antibiotics including cephalosporins and cephamycins. It is one of the most common 4-membered ring heterocycle. Produced by actinomycetes, cephamycins were found to display antibacterial activity against a wide range of bacteria, including those resistant to penicillin and cephalosporins. The antimicrobial properties of Cephem include the attachment to certain penicillin-binding proteins that are involved in the production of cell walls of bacteria.
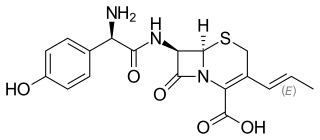
Cefprozil is a second-generation cephalosporin antibiotic. Originally discovered in 1983, and approved in 1992, it was sold under the tradename Cefzil by Bristol Meyers Squibb until 2010 when the brand name version was discontinued. It continues to be available from various companies in its generic form. It is used in the treatment of pharyngitis, tonsillitis, ear infections, acute sinusitis, bacterial exacerbation of chronic bronchitis, and skin and skin structure infections. It is currently available as a tablet and as a liquid suspension.

Alkylresorcinols (ARs), also known as resorcinolic lipids, are amphiphilic phenolic lipids characterised by a non-polar odd-numbered alkyl side chain with up to 27 carbon atoms attached to a polar resorcinol (1,3-dihydroxybenzene) ring.
Streptomyces spectabilis is a bacterium species from the genus of Streptomyces. Streptomyces spectabilis produces hangtaimycin, gentamicin, kanamycin, neomycin B, sisomycin, tobramycin, paromomycin, spectinabilin, spectinomycin, aminocyclitol, actinospectacin, prodigiosine and the streptovaricin complex.

Hachimycin, also known as trichomycin, is a polyene macrolide antibiotic, antiprotozoal, and antifungal derived from streptomyces. It was first described in 1950, and in most research cases have been used for gynecological infections.

Aquayamycin is an anthraquinone derivative. It is an inhibitor of the enzyme tyrosine hydroxylase.
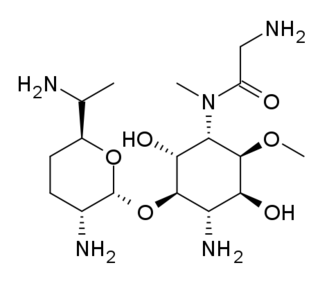
Astromicin (INN)(also frequently referenced in scientific journal articles as compounds Fortimicin A/B ) is an aminoglycoside antibiotic. Synthesized from Micromonospora olivasterospora(also named with additional o in olivoasterospora).

Kynapcin is a general name for a number of dibenzofuranyl derivatives of the molecule polyozellin, present in the fungus Polyozellus multiplex. Like polyozellin, some kynapcins inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins including amyloid precursor protein. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Several kynapcins have been found in P. multiplex, each with different chemical properties, including kynapcin-12, kynapcin-13 and -28, and -24. A total synthesis of kynapcin-24 was achieved in 2009.

Polyozellin is a chemical which occurs in the mushroom Polyozellus multiplex. It inhibits prolyl endopeptidase, an enzyme that has a role in processing proteins in Alzheimer's disease. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Structurally related dibenzofuranyl derivatives of polyozellin are known as kynapcins.
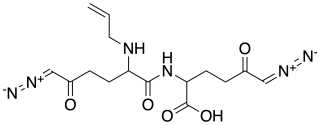
Alazopeptin is an antibiotic, with moderate anti-trypanosomal and antitumor activity. It was originally isolated from Streptacidiphilus griseoplanus, sourced from soil near Williamsburg, Iowa. It is also isolated from Kitasatospora azatica. It is still largely produced via fermentation broths of that organism. Structurally, alazopeptin is a tripeptide and contains 2 molecules of 6-diazo-5-oxo-L-norleucine and one molecule of L-alanine. In 2021 the biosynthetic pathway of alazopeptin was elucidated.

Streptomyces hygroscopicus is a bacterial species in the genus Streptomyces. It was first described by Hans Laurits Jensen in 1931.
Adipostatin A is an alkylresorcinol, a type of phenolic lipids composed of long aliphatic chains and phenolic rings. Chemically, it is similar in structure to urushiol, the irritant found in poison ivy.
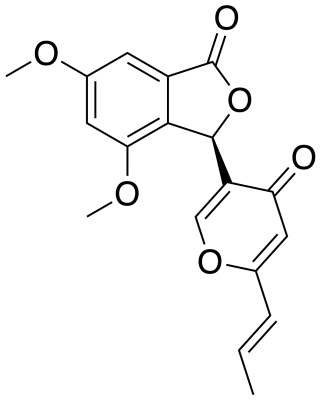
Vermistatin is an organic compound and a metabolite of mine-dwelling Penicillium vermiculatum found in Berkeley Pit Lake, Butte, Montana. Penisimplicissin is a vermistatin analog with anticancer activity.

Kidamycin is an anthracycline antibiotic with anticancer activity. It was first synthesized from a strain of streptomyces bacteria isolated from a soil sample. In clinical trials, Kindamycin showed high effect against gram positive bacteria as well as multiple cancer models including Ehrlich ascites carcinoma, Sarcoma 180, NF-sarcoma, and Yoshida sarcoma.
Naphthomycins are a group of closely related antimicrobial chemical compounds isolated from Streptomyces. They are considered a subclass of ansamycins.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Streptomyces cattleya is a Gram-positive bacterium which makes cephamycin, penicillin and thienamycin. The bacterium expresses a fluorinase enzyme, and the organism has been used to understand the biosynthesis of fluoroacetate and the antibacterial 4-fluoro-L-threonine. The γ-Glu-βes pathway to biosynthesis of non-traditional amino acids β-ethynylserine (βes) and L-propargylglycine (Pra) was first characterized in this species.
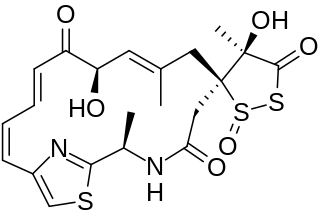
Leinamycin is an 18-membered macrolactam produced by several species of Streptomyces atroolivaceus. This macrolactam has also been shown to exhibit antitumor properties as well as antimicrobial properties against gram-positive and gram-negative bacteria. The presence of a spiro-fused 1,3-dioxo-1,2-dithiolane moiety was a unique structural property at the time of this compound's discovery and it plays an important role in leinamycin's antitumor and antibacterial properties due to its ability to inhibit DNA synthesis.
Micromonospora citrea is an endophytic actinomycete. It produces citreamicins, several types of antibacterial antibiotics.
Micromonospora sagamiensis is an endophytic actinomycete. It produces sagamicin, an aminoglycoside antibiotic, as well as several mutational variants. Its cell wall contains only D-alanine.













