Experimental cancer treatments are mainstream medical therapies intended to treat cancer by improving on, supplementing or replacing conventional methods. However, researchers are still trying to determine whether these treatments are safe and effective treatments. Experimental cancer treatments are normally available only to people who participate in formal research programs, which are called clinical trials. Occasionally, a seriously ill person may be able to access an experimental drug through an expanded access program. Some of the treatments have regulatory approval for treating other conditions. Health insurance and publicly funded health care programs normally refuse to pay for experimental cancer treatments.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.

Tumor hypoxia is the situation where tumor cells have been deprived of oxygen. As a tumor grows, it rapidly outgrows its blood supply, leaving portions of the tumor with regions where the oxygen concentration is significantly lower than in healthy tissues. Hypoxic microenvironements in solid tumors are a result of available oxygen being consumed within 70 to 150 μm of tumour vasculature by rapidly proliferating tumor cells thus limiting the amount of oxygen available to diffuse further into the tumor tissue. In order to support continuous growth and proliferation in challenging hypoxic environments, cancer cells are found to alter their metabolism. Furthermore, hypoxia is known to change cell behavior and is associated with extracellular matrix remodeling and increased migratory and metastatic behavior.
In the field of genetics, a suicide gene is a gene that will cause a cell to kill itself through the process of apoptosis. Activation of a suicide gene can cause death through a variety of pathways, but one important cellular "switch" to induce apoptosis is the p53 protein. Stimulation or introduction of suicide genes is a potential way of treating cancer or other proliferative diseases.
A prodrug is a pharmacologically inactive medication or compound that, after intake, is metabolized into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug is absorbed, distributed, metabolized, and excreted (ADME).

Thymidine kinase is an enzyme, a phosphotransferase : 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21. It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2. Certain viruses also have genetic information for expression of viral thymidine kinases. Thymidine kinase catalyzes the reaction:

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune system responses. Oncolytic viruses also have the ability to affect the tumor micro-environment in multiple ways.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
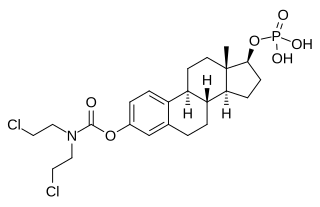
Estramustine phosphate (EMP), also known as estradiol normustine phosphate and sold under the brand names Emcyt and Estracyt, is a dual estrogen and chemotherapy medication which is used in the treatment of prostate cancer in men. It is taken multiple times a day by mouth or by injection into a vein.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies are used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Minretumomab (CC49) is a mouse monoclonal antibody that was designed for the treatment of cancers that express the TAG-72 antigen. This includes breast, colon, lung, and pancreatic cancers. Apparently, it never got past Phase I clinical trials for this purpose.

Meir Wilchek is an Israeli biochemist. He is a professor at the Weizmann Institute of Science.
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by Clostridium botulinum and less frequently by other Clostridium species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxin. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biology.
Cancer cells are cells that divide continually, forming solid tumors or flooding the blood or lymph with abnormal cells. Cell division is a normal process used by the body for growth and repair. A parent cell divides to form two daughter cells, and these daughter cells are used to build new tissue or to replace cells that have died because of aging or damage. Healthy cells stop dividing when there is no longer a need for more daughter cells, but cancer cells continue to produce copies. They are also able to spread from one part of the body to another in a process known as metastasis.
The duocarmycins are members of a series of related natural products first isolated from Streptomyces bacteria in 1978. They are notable for their extreme cytotoxicity and thus represent a class of exceptionally potent antitumour antibiotics.
Tegafur/gimeracil/oteracil, sold under the brand name Teysuno among others is a fixed-dose combination medication used for the treatment of advanced gastric cancer when used in combination with cisplatin, and also for the treatment of head and neck cancer, colorectal cancer, non–small-cell lung, breast, pancreatic, and biliary tract cancers.
Gold nanoparticles in chemotherapy and radiotherapy is the use of colloidal gold in therapeutic treatments, often for cancer or arthritis. Gold nanoparticle technology shows promise in the advancement of cancer treatments. Some of the properties that gold nanoparticles possess, such as small size, non-toxicity and non-immunogenicity make these molecules useful candidates for targeted drug delivery systems. With tumor-targeting delivery vectors becoming smaller, the ability to by-pass the natural barriers and obstacles of the body becomes more probable. To increase specificity and likelihood of drug delivery, tumor specific ligands may be grafted onto the particles along with the chemotherapeutic drug molecules, to allow these molecules to circulate throughout the tumor without being redistributed into the body.

Melphalan flufenamide, sold under the brand names Pepaxto and Pepaxti, is an anticancer medication used to treat multiple myeloma.
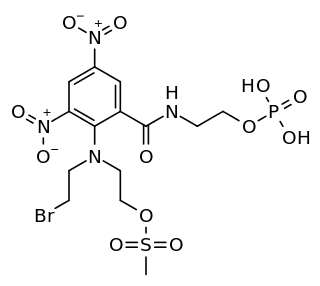
PR-104 is a drug from the class of hypoxia-activated prodrugs (HAPs), which is being researched as a potential anti-cancer therapeutic agent. It is a phosphate ester “pre-prodrug” that is rapidly converted to the HAP PR-104A in the body. PR-104A is in turn metabolised to reactive nitrogen mustard DNA crosslinking agents in hypoxic tissues such as found in solid tumours. Following initial clinical studies, it was discovered that PR-104A is also activated by the enzyme AKR1C3, independently of hypoxia. Hypoxia in the bone marrow of patients with leukaemia, and high activity of AKR1C3 in some leukaemia subtypes has led to interest in clinical trials of PR-104 in relapsed refractory acute leukaemias.










