Related Research Articles

Dendrimers are highly ordered, branched polymeric molecules. The name comes from the Czech word dheidri (deidri) which translates to "tree". Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.

Anthracyclines is a class of drugs used in cancer chemotherapy that are extracted from Streptomyces bacterium. These compounds are used to treat many cancers, including leukemias, lymphomas, breast, stomach, uterine, ovarian, bladder cancer, and lung cancers. The first anthracycline discovered was daunorubicin, which is produced naturally by Streptomyces peucetius, a species of actinobacteria. Clinically the most important anthracyclines are doxorubicin, daunorubicin, epirubicin and idarubicin.
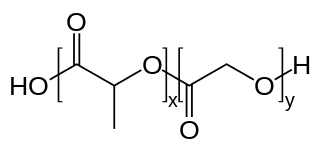
PLGA, PLG, or poly(lactic-co-glycolic acid) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.
The enhanced permeability and retention (EPR) effect is a controversial concept by which molecules of certain sizes tend to accumulate in tumor tissue much more than they do in normal tissues. The general explanation that is given for this phenomenon is that, in order for tumor cells to grow quickly, they must stimulate the production of blood vessels. VEGF and other growth factors are involved in cancer angiogenesis. Tumor cell aggregates as small as 150–200 μm, start to become dependent on blood supply carried out by neovasculature for their nutritional and oxygen supply. These newly formed tumor vessels are usually abnormal in form and architecture. They are poorly aligned defective endothelial cells with wide fenestrations, lacking a smooth muscle layer, or innervation with a wider lumen, and impaired functional receptors for angiotensin II. Furthermore, tumor tissues usually lack effective lymphatic drainage. All of these factors lead to abnormal molecular and fluid transport dynamics, especially for macromolecular drugs. This phenomenon is referred to as the "enhanced permeability and retention (EPR) effect" of macromolecules and lipids in solid tumors. The EPR effect is further enhanced by many pathophysiological factors involved in enhancement of the extravasation of macromolecules in solid tumor tissues. For instance, bradykinin, nitric oxide / peroxynitrite, prostaglandins, vascular permeability factor, tumor necrosis factor and others. One factor that leads to the increased retention is the lack of lymphatics around the tumor region which would filter out such particles under normal conditions.
A drug carrier is any substrate used in the process of drug delivery which serves to improve the selectivity, effectiveness, and/or safety of drug administration. Drug carriers are primarily used to control the release of a drug into systemic circulation. This can be accomplished either by slow release of the drug over a long period of time or by triggered release at the drug's target by some stimulus, such as changes in pH, application of heat, and activation by light. Drug carriers are also used to improve the pharmacokinetic properties, specifically the bioavailability, of many drugs with poor water solubility and/or membrane permeability.
Targeted drug delivery, sometimes called smart drug delivery, is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to others. This means of delivery is largely founded on nanomedicine, which plans to employ nanoparticle-mediated drug delivery in order to combat the downfalls of conventional drug delivery. These nanoparticles would be loaded with drugs and targeted to specific parts of the body where there is solely diseased tissue, thereby avoiding interaction with healthy tissue. The goal of a targeted drug delivery system is to prolong, localize, target and have a protected drug interaction with the diseased tissue. The conventional drug delivery system is the absorption of the drug across a biological membrane, whereas the targeted release system releases the drug in a dosage form. The advantages to the targeted release system is the reduction in the frequency of the dosages taken by the patient, having a more uniform effect of the drug, reduction of drug side-effects, and reduced fluctuation in circulating drug levels. The disadvantage of the system is high cost, which makes productivity more difficult and the reduced ability to adjust the dosages.
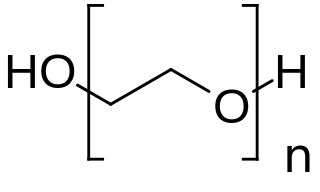
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated.

Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.
CRLX101 is a novel approach to cancer chemotherapy that is under investigation in human trials. It is an example of a nanomedicine.
A nanogel is a nanoparticle composed of a hydrogel—a crosslinked hydrophilic polymer network. Nanogels are most often composed of synthetic polymers or biopolymers which are chemically or physically crosslinked. Nanogels are usually in the tens to hundreds of nanometers in diameter. Like hydrogels, nanogels have low density of macromolecules and their pores can be filled with small molecules or macromolecules, and their properties, such as swelling, degradation, and chemical functionality, can be controlled.

Antibody-drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs.

Jindřich (Henry) Kopeček was born in Strakonice, Czech Republic as the son of Jan and Herta Zita (Krombholz) Kopecek. He holds the title of Distinguished Professor in both the Pharmaceutical Chemistry and Biomedical Engineering departments at the University of Utah in Salt Lake City, Utah. Kopeček is also an Honorary Professor at Sichuan University in Chengdu, China. His research focus is geared towards biorecognition of macromolecules, bioconjugate chemistry, drug delivery systems, self-assembled biomaterials, and drug-free macromolecular therapeutics.

N-(2-Hydroxypropyl)methacrylamide or HPMA is the monomer used to make the polymer poly(N- methacrylamide).

A nanocarrier is nanomaterial being used as a transport module for another substance, such as a drug. Commonly used nanocarriers include micelles, polymers, carbon-based materials, liposomes and other substances. Nanocarriers are currently being studied for their use in drug delivery and their unique characteristics demonstrate potential use in chemotherapy.
Directed enzyme prodrug therapy (DEPT) uses enzymes artificially introduced into the body to convert prodrugs, which have no or poor biologically activity, to the active form in the desired location within the body. Many chemotherapy drugs for cancer lack tumour specificity and the doses required to reach therapeutic levels in the tumour are often toxic to other tissues. DEPT strategies are an experimental method of reducing the systemic toxicity of a drug, by achieving high levels of the active drug only at the desired site. This article describes the variations of DEPT technology.
Nanoparticles for drug delivery to the brain is a method for transporting drug molecules across the blood–brain barrier (BBB) using nanoparticles. These drugs cross the BBB and deliver pharmaceuticals to the brain for therapeutic treatment of neurological disorders. These disorders include Parkinson's disease, Alzheimer's disease, schizophrenia, depression, and brain tumors. Part of the difficulty in finding cures for these central nervous system (CNS) disorders is that there is yet no truly efficient delivery method for drugs to cross the BBB. Antibiotics, antineoplastic agents, and a variety of CNS-active drugs, especially neuropeptides, are a few examples of molecules that cannot pass the BBB alone. With the aid of nanoparticle delivery systems, however, studies have shown that some drugs can now cross the BBB, and even exhibit lower toxicity and decrease adverse effects throughout the body. Toxicity is an important concept for pharmacology because high toxicity levels in the body could be detrimental to the patient by affecting other organs and disrupting their function. Further, the BBB is not the only physiological barrier for drug delivery to the brain. Other biological factors influence how drugs are transported throughout the body and how they target specific locations for action. Some of these pathophysiological factors include blood flow alterations, edema and increased intracranial pressure, metabolic perturbations, and altered gene expression and protein synthesis. Though there exist many obstacles that make developing a robust delivery system difficult, nanoparticles provide a promising mechanism for drug transport to the CNS.
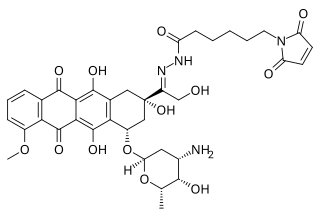
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid sensitive linker.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.

Blanka Říhová is a Czech immunologist. Her research involves the development of targeted drug delivery methods for cancer. She is the former director at the Institute of Microbiology of the Czech Academy of Sciences. In 2018 Říhová was made President of the Learned Society of the Czech Republic.
Hamid Ghandehari, born in Iran, is a drug delivery research scientist, and a professor in the Departments of Pharmaceutics and Pharmaceutical Chemistry and Biomedical Engineering at the University of Utah. His research is focused in recombinant polymers for drug and gene delivery, nanotoxicology of dendritic and inorganic constructs, water-soluble polymers for targeted delivery and poly(amidoamine) dendrimers for oral delivery.
References
- ↑ Feng, Q; Tong, R (2016). "Anticancer nanoparticulate polymer‐drug conjugate". Bioengineering & Translational Medicine. American Institute of Chemical Engineers. 1 (3): 277–296. doi:10.1002/btm2.10033. PMC 5689533 . PMID 29313017.
- ↑ Bertrand, Nicolas; Leroux, Jean-Christope (2012). "The Journey of a Drug-carrier in the Body: An Anatomo-physiological Perspective". Institute of Pharmaceutical Sciences. 161 (2): 152–63. doi:10.1016/j.jconrel.2011.09.098. PMID 22001607.
- ↑ Chau, Ying (2005). Targeted drug delivery by novel polymer-drug conjugates containing linkers cleavable by disease-associated enzymes (Thesis). hdl:1721.1/32332.
- ↑ Duncan, R; Vincent, M J (2005). "Polymer-drug Conjugates: Towards a Novel Approach for the Treatment of Endrocrine-related Cancer". Endocrine-Related Cancer . Bioscientifica. 12: 189–199. doi:10.1677/erc.1.01045. PMID 16113096.
- ↑ Khandare, Jayant; Minko, Tamara (April 2006). "Polymer–drug conjugates: Progress in polymeric prodrugs". Progress in Polymer Science. 31 (4): 359–397. doi:10.1016/j.progpolymsci.2005.09.004.
- ↑ Vicent, Maria J. (June 2007). "Polymer-drug conjugates as modulators of cellular apoptosis". The AAPS Journal. 9 (2): E200–E207. doi:10.1208/aapsj0902022. PMC 2751409 . PMID 17907762.