Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).

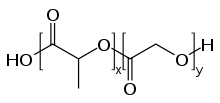
Polyglycolide or poly(glycolic acid) (PGA), also spelled as polyglycolic acid, is a biodegradable, thermoplastic polymer and the simplest linear, aliphatic polyester. It can be prepared starting from glycolic acid by means of polycondensation or ring-opening polymerization. PGA has been known since 1954 as a tough fiber-forming polymer. Owing to its hydrolytic instability, however, its use has initially been limited. Currently polyglycolide and its copolymers (poly(lactic-co-glycolic acid) with lactic acid, poly(glycolide-co-caprolactone) with ε-caprolactone and poly (glycolide-co-trimethylene carbonate) with trimethylene carbonate) are widely used as a material for the synthesis of absorbable sutures and are being evaluated in the biomedical field.

Polycaprolactone (PCL) is a synthetic, semi-crystalline, biodegradable polyester with a melting point of about 60 °C and a glass transition temperature of about −60 °C. The most common use of polycaprolactone is in the production of speciality polyurethanes. Polycaprolactones impart good resistance to water, oil, solvent and chlorine to the polyurethane produced.
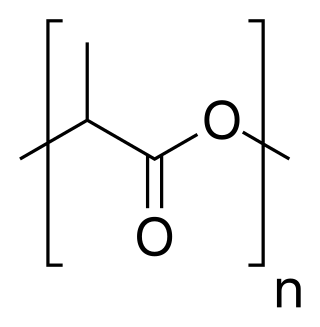
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.

Organ printing utilizes techniques similar to conventional 3D printing where a computer model is fed into a printer that lays down successive layers of plastics or wax until a 3D object is produced. In the case of organ printing, the material being used by the printer is a biocompatible plastic. The biocompatible plastic forms a scaffold that acts as the skeleton for the organ that is being printed. As the plastic is being laid down, it is also seeded with human cells from the patient's organ that is being printed for. After printing, the organ is transferred to an incubation chamber to give the cells time to grow. After a sufficient amount of time, the organ is implanted into the patient.

Nanofibers are fibers with diameters in the nanometer range. Nanofibers can be generated from different polymers and hence have different physical properties and application potentials. Examples of natural polymers include collagen, cellulose, silk fibroin, keratin, gelatin and polysaccharides such as chitosan and alginate. Examples of synthetic polymers include poly(lactic acid) (PLA), polycaprolactone (PCL), polyurethane (PU), poly(lactic-co-glycolic acid) (PLGA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly(ethylene-co-vinylacetate) (PEVA). Polymer chains are connected via covalent bonds. The diameters of nanofibers depend on the type of polymer used and the method of production. All polymer nanofibers are unique for their large surface area-to-volume ratio, high porosity, appreciable mechanical strength, and flexibility in functionalization compared to their microfiber counterparts.
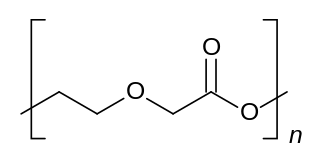
Polydioxanone or poly-p-dioxanone is a colorless, crystalline, biodegradable synthetic polymer.
Polyanhydrides are a class of biodegradable polymers characterized by anhydride bonds that connect repeat units of the polymer backbone chain. Their main application is in the medical device and pharmaceutical industry. In vivo, polyanhydrides degrade into non-toxic diacid monomers that can be metabolized and eliminated from the body. Owing to their safe degradation products, polyanhydrides are considered to be biocompatible.
A nerve guidance conduit is an artificial means of guiding axonal regrowth to facilitate nerve regeneration and is one of several clinical treatments for nerve injuries. When direct suturing of the two stumps of a severed nerve cannot be accomplished without tension, the standard clinical treatment for peripheral nerve injuries is autologous nerve grafting. Due to the limited availability of donor tissue and functional recovery in autologous nerve grafting, neural tissue engineering research has focused on the development of bioartificial nerve guidance conduits as an alternative treatment, especially for large defects. Similar techniques are also being explored for nerve repair in the spinal cord but nerve regeneration in the central nervous system poses a greater challenge because its axons do not regenerate appreciably in their native environment.
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.
Many opportunities exist for the application of synthetic biodegradable polymers in the biomedical area particularly in the fields of tissue engineering and controlled drug delivery. Degradation is important in biomedicine for many reasons. Degradation of the polymeric implant means surgical intervention may not be required in order to remove the implant at the end of its functional life, eliminating the need for a second surgery. In tissue engineering, biodegradable polymers can be designed such to approximate tissues, providing a polymer scaffold that can withstand mechanical stresses, provide a suitable surface for cell attachment and growth, and degrade at a rate that allows the load to be transferred to the new tissue. In the field of controlled drug delivery, biodegradable polymers offer tremendous potential either as a drug delivery system alone or in conjunction to functioning as a medical device.
Nano-scaffolding or nanoscaffolding is a medical process used to regrow tissue and bone, including limbs and organs. The nano-scaffold is a three-dimensional structure composed of polymer fibers very small that are scaled from a Nanometer scale. Developed by the American military, the medical technology uses a microscopic apparatus made of fine polymer fibers called a scaffold. Damaged cells grip to the scaffold and begin to rebuild missing bone and tissue through tiny holes in the scaffold. As tissue grows, the scaffold is absorbed into the body and disappears completely.
Heart nanotechnology is the "Engineering of functional systems at the molecular scale".

Poly(3-hydroxybutyrate-co-3-hydroxyvalerate), commonly known as PHBV, is a polyhydroxyalkanoate-type polymer. It is biodegradable, nontoxic, biocompatible plastic produced naturally by bacteria and a good alternative for many non-biodegradable synthetic polymers. It is a thermoplastic linear aliphatic polyester. It is obtained by the copolymerization of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid. PHBV is used in speciality packaging, orthopedic devices and in controlled release of drugs. PHBV undergoes bacterial degradation in the environment.
Polymer-drug conjugates are nano-medicine products under development for cancer diagnosis and treatment. There are more than 10 anticancer conjugates in clinical development. Polymer-drug conjugates are drug molecules held in polymer molecules, which act as the delivery system for the drug. Polymer drugs have passed multidrug resistance (MDR) testing and hence may become a viable treatment for endocrine-related cancers. A cocktail of pendant drugs could be delivered by water-soluble polymer platforms. The physical and chemical properties of the polymers used in polymer-drug conjugates are specially synthesized to flow through the kidneys and liver without being filtered out, allowing the drugs to be used more effectively. Traditional polymers used in polymer-drug conjugates can be degraded through enzymatic activity and acidity. Polymers are now being synthesized to be sensitive to specific enzymes that are apparent in diseased tissue. The drugs remain attached to the polymer and are not activated until the enzymes associated with the diseased tissue are present. This process significantly minimizes damage to healthy tissue.
Poly(ethylene adipate) or PEA is an aliphatic polyester. It is most commonly synthesized from a polycondensation reaction between ethylene glycol and adipic acid. PEA has been studied as it is biodegradable through a variety of mechanisms and also fairly inexpensive compared to other polymers. Its lower molecular weight compared to many polymers aids in its biodegradability.
Polyorthoesters are polymers with the general structure –[–R–O–C(R1, OR2)–O–R3–]n– whereas the residue R2 can also be part of a heterocyclic ring with the residue R. Polyorthoesters are formed by transesterification of orthoesters with diols or by polyaddition between a diol and a diketene acetal, such as 3,9-diethylidene-2,4,8,10-tetraoxaspiro[5.5]undecane.

Acetalated dextran is a biodegradable polymer based on dextran that has acetal modified hydroxyl groups. After synthesis, the hydrophilic polysaccharide dextran is rendered insoluble in water, but soluble in organic solvents. This allows it to be processed in the same manner as many polyesters, like poly(lactic-co-glycolic acid), through processes like solvent evaporation and emulsion. Acetalated dextran is structurally different from acetylated dextran.
Chitosan-poly is a composite that has been increasingly used to create chitosan-poly(acrylic acid) nanoparticles. More recently, various composite forms have come out with poly(acrylic acid) being synthesized with chitosan which is often used in a variety of drug delivery processes. Chitosan which already features strong biodegradability and biocompatibility nature can be merged with polyacrylic acid to create hybrid nanoparticles that allow for greater adhesion qualities as well as promote the biocompatibility and homeostasis nature of chitosan poly(acrylic acid) complex. The synthesis of this material is essential in various applications and can allow for the creation of nanoparticles to facilitate a variety of dispersal and release behaviors and its ability to encapsulate a multitude of various drugs and particles.

Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate (1,3-propylene carbonate or 1,3-Dioxan-2-one). Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255°C (at 760 mmHg). TMC is originally synthesized from 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.