
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.

Anthracyclines are a class of drugs used in cancer chemotherapy that are extracted from Streptomyces bacterium. These compounds are used to treat many cancers, including leukemias, lymphomas, breast, stomach, uterine, ovarian, bladder cancer, and lung cancers. The first anthracycline discovered was daunorubicin, which is produced naturally by Streptomyces peucetius, a species of Actinomycetota. Clinically the most important anthracyclines are doxorubicin, daunorubicin, epirubicin and idarubicin.
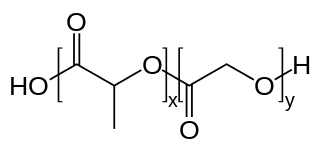
PLGA, PLG, or poly(lactic-co-glycolic acid) is a copolymer which is used in a host of Food and Drug Administration (FDA) approved therapeutic devices, owing to its biodegradability and biocompatibility. PLGA is synthesized by means of ring-opening co-polymerization of two different monomers, the cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid. Polymers can be synthesized as either random or block copolymers thereby imparting additional polymer properties. Common catalysts used in the preparation of this polymer include tin(II) 2-ethylhexanoate, tin(II) alkoxides, or aluminum isopropoxide. During polymerization, successive monomeric units are linked together in PLGA by ester linkages, thus yielding a linear, aliphatic polyester as a product.

The enhanced permeability and retention (EPR) effect is a controversial concept by which molecules of certain sizes tend to accumulate in tumor tissue much more than they do in normal tissues. The general explanation that is given for this phenomenon is that, in order for tumor cells to grow quickly, they must stimulate the production of blood vessels. VEGF and other growth factors are involved in cancer angiogenesis. Tumor cell aggregates as small as 150–200 μm, start to become dependent on blood supply carried out by neovasculature for their nutritional and oxygen supply. These newly formed tumor vessels are usually abnormal in form and architecture. They are poorly aligned defective endothelial cells with wide fenestrations, lacking a smooth muscle layer, or innervation with a wider lumen, and impaired functional receptors for angiotensin II. Furthermore, tumor tissues usually lack effective lymphatic drainage. All of these factors lead to abnormal molecular and fluid transport dynamics, especially for macromolecular drugs. This phenomenon is referred to as the "enhanced permeability and retention (EPR) effect" of macromolecules and lipids in solid tumors. The EPR effect is further enhanced by many pathophysiological factors involved in enhancement of the extravasation of macromolecules in solid tumor tissues. For instance, bradykinin, nitric oxide / peroxynitrite, prostaglandins, vascular permeability factor, tumor necrosis factor and others. One factor that leads to the increased retention is the lack of lymphatics around the tumor region which would filter out such particles under normal conditions.
Poloxamers are nonionic triblock copolymers composed of a central hydrophobic chain of polyoxypropylene flanked by two hydrophilic chains of polyoxyethylene. The word poloxamer was coined by BASF inventor, Irving Schmolka, who received the patent for these materials in 1973. Poloxamers are also known by the trade names Pluronic, Kolliphor, and Synperonic.
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.
Thiolated polymers – designated thiomers – are functional polymers used in biotechnology product development with the intention to prolong mucosal drug residence time and to enhance absorption of drugs. The name thiomer was coined by Andreas Bernkop-Schnürch in 2000. Thiomers have thiol bearing side chains. Sulfhydryl ligands of low molecular mass are covalently bound to a polymeric backbone consisting of mainly biodegradable polymers, such as chitosan, hyaluronic acid, cellulose derivatives, pullulan, starch, gelatin, polyacrylates, cyclodextrins, or silicones.
Small-angle X-ray scattering (SAXS) is a small-angle scattering technique by which nanoscale density differences in a sample can be quantified. This means that it can determine nanoparticle size distributions, resolve the size and shape of (monodisperse) macromolecules, determine pore sizes, characteristic distances of partially ordered materials, and much more. This is achieved by analyzing the elastic scattering behaviour of X-rays when travelling through the material, recording their scattering at small angles. It belongs to the family of small-angle scattering (SAS) techniques along with small-angle neutron scattering, and is typically done using hard X-rays with a wavelength of 0.07 – 0.2 nm. Depending on the angular range in which a clear scattering signal can be recorded, SAXS is capable of delivering structural information of dimensions between 1 and 100 nm, and of repeat distances in partially ordered systems of up to 150 nm. USAXS can resolve even larger dimensions, as the smaller the recorded angle, the larger the object dimensions that are probed.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit drastic and discontinuous changes in their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, either an upper critical solution temperature (UCST) or a lower critical solution temperature (LCST) exists.
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis. Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms. Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.

Jindřich Henry Kopeček was born in Strakonice, Czech Republic, as the son of Jan and Herta Zita (Krombholz) Kopeček. He is distinguished professor of pharmaceutical chemistry and distinguished professor of biomedical engineering at the University of Utah in Salt Lake City, Utah. Kopeček is also an honorary professor at Sichuan University in Chengdu, China. His research focuses on biorecognition of macromolecules, bioconjugate chemistry, drug delivery systems, self-assembled biomaterials, and drug-free macromolecular therapeutics.
Polymer-drug conjugates are nano-medicine products under development for cancer diagnosis and treatment. There are more than 10 anticancer conjugates in clinical development. Polymer-drug conjugates are drug molecules held in polymer molecules, which act as the delivery system for the drug. Polymer drugs have passed multidrug resistance (MDR) testing and hence may become a viable treatment for endocrine-related cancers. A cocktail of pendant drugs could be delivered by water-soluble polymer platforms. The physical and chemical properties of the polymers used in polymer-drug conjugates are specially synthesized to flow through the kidneys and liver without being filtered out, allowing the drugs to be used more effectively. Traditional polymers used in polymer-drug conjugates can be degraded through enzymatic activity and acidity. Polymers are now being synthesized to be sensitive to specific enzymes that are apparent in diseased tissue. The drugs remain attached to the polymer and are not activated until the enzymes associated with the diseased tissue are present. This process significantly minimizes damage to healthy tissue.
Directed enzyme prodrug therapy (DEPT) uses enzymes artificially introduced into the body to convert prodrugs, which have no or poor biologically activity, to the active form in the desired location within the body. Many chemotherapy drugs for cancer lack tumour specificity and the doses required to reach therapeutic levels in the tumour are often toxic to other tissues. DEPT strategies are an experimental method of reducing the systemic toxicity of a drug, by achieving high levels of the active drug only at the desired site. This article describes the variations of DEPT technology.

Andreas Bernkop-Schnürch is an Austrian scientist and entrepreneur. He is Head of the Department of Pharmaceutical Technology, Institute of Pharmacy, University of Innsbruck. His research centers on the areas of drug delivery, dosage forms, controlled release, bionanotechnology, polymer engineering and tissue engineering. He is the inventor of several technologies such as thiolated polymers for that he coined the name thiomers in 2000 and phosphatase triggered charge-converting nanoparticles for mucosal drug delivery. Since 2014 he is on the scientific advisory board of the Nicotine Science Center, Denmark. From 2016 to 2018 he served as a member of the Scientific Committee of the Innovative Medicines Initiative (IMI) of the European Union in Brussels giving advice on scientific priorities to be included in the Strategic Research Agenda for Horizon 2020. He is member of the editorial board of numerous pharmaceutical journals including International Journal of Pharmaceutics, Journal of Drug Delivery Science and Technology, Scientia Pharmaceutica, Drug Development and Industrial Pharmacy and European Journal of Pharmaceutics and Biopharmaceutis. Andreas Bernkop-Schnürch is the founder of Thiomatrix Forschungs- und Beratungs GmbH, Mucobiomer Biotechnologische Forschungs- und Entwicklungs GmbH and Green River Polymers Forschungs und Entwicklungs GmbH. Since 2022 he is listed as a Highly Cited Researcher of the Institute of Scientific Information.
Christine Allen is a Canadian professor and the first associate vice-president and vice-provost for strategic initiatives at the University of Toronto. She served formerly as interim dean of the university's Leslie Dan Faculty of Pharmacy. She is co-founder of Nanovista, a company focused on imaging of tumors. She also works as the associate editor of Molecular Pharmaceutics.

Blanka Říhová is a Czech immunologist. Her research involves the development of targeted drug delivery methods for cancer. She is the former director at the Institute of Microbiology of the Czech Academy of Sciences. In 2018 Říhová was made President of the Learned Society of the Czech Republic.
Hamid Ghandehari is an Iranian-American drug delivery research scientist, and a professor in the Departments of Pharmaceutics and Pharmaceutical Chemistry and Biomedical Engineering at the University of Utah. His research is focused in recombinant polymers for drug and gene delivery, nanotoxicology of dendritic and inorganic constructs, water-soluble polymers for targeted delivery and poly(amidoamine) dendrimers for oral delivery.
Pullulan bioconjugates are systems that use pullulan as a scaffold to attach biological materials to, such as drugs. These systems can be used to enhance the delivery of drugs to specific environments or the mechanism of delivery. These systems can be used in order to deliver drugs in response to stimuli, create a more controlled and sustained release, and provide a more targeted delivery of certain drugs.
pH-responsive tumor-targeted drug delivery is a specialized form of targeted drug delivery that utilizes nanoparticles to deliver therapeutic drugs directly to cancerous tumor tissue while minimizing its interaction with healthy tissue. Scientists have used drug delivery as a way to modify the pharmacokinetics and targeted action of a drug by combining it with various excipients, drug carriers, and medical devices. These drug delivery systems have been created to react to the pH environment of diseased or cancerous tissues, triggering structural and chemical changes within the drug delivery system. This form of targeted drug delivery is to localize drug delivery, prolongs the drug's effect, and protect the drug from being broken down or eliminated by the body before it reaches the tumor.
Coiled-coil drug delivery systems refer to drug delivery systems utilizing coiled-coil motifs capable of delivering disease-treating therapies, imaging agents, and vaccines to patients systemically or specifically. These systems are a form of peptide therapeutics and are capable of being engineered and finely tuned into different types of drug delivery vehicles based on the specific application required. The goal of a coiled-coil drug delivery system is to deliver cargo such as medication, imaging agents, biological molecules, or vaccines efficiently and specifically, in order to maximize the therapeutic efficacy and minimize unwanted side effects. This is achieved through fine-tuning the factors affecting the coiled coil’s oligomerization, resulting in modular systems that are highly specific for the intended application.










