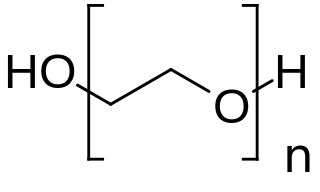
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.

Lipid-based nanoparticles are very small spherical particles composed of lipids. They are a novel pharmaceutical drug delivery system, and a novel pharmaceutical formulation. There are many subclasses of lipid-based nanoparticles such as: lipid nanoparticles (LNPs), solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs).

Moderna, Inc. is an American pharmaceutical and biotechnology company based in Cambridge, Massachusetts, that focuses on RNA therapeutics, primarily mRNA vaccines. These vaccines use a copy of a molecule called messenger RNA (mRNA) to carry instructions for proteins to produce an immune response. The company's name is derived from the terms "modified", "RNA", and "modern".

Arcturus Therapeutics Holdings Inc. is an American RNA medicines biotechnology company focused on the discovery, development and commercialization of therapeutics for rare diseases and infectious diseases. Arcturus has developed proprietary lipid nanoparticle RNA therapeutics for nucleic acid medicines including small interfering RNA (siRNA), messenger RNA (mRNA), gene editing RNA, DNA, antisense oligonucleotides, and microRNA.

An mRNAvaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. The vaccine delivers molecules of antigen-encoding mRNA into cells, which use the designed mRNA as a blueprint to build foreign protein that would normally be produced by a pathogen or by a cancer cell. These protein molecules stimulate an adaptive immune response that teaches the body to identify and destroy the corresponding pathogen or cancer cells. The mRNA is delivered by a co-formulation of the RNA encapsulated in lipid nanoparticles that protect the RNA strands and help their absorption into the cells.

A COVID‑19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID‑19).

The Moderna COVID‑19 vaccine, sold under the brand name Spikevax, is a COVID-19 vaccine developed by the American company Moderna, the United States National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). Depending on the jurisdiction, it is authorized for use in humans aged six months, twelve years, or eighteen years and older. It provides protection against COVID-19, which is caused by infection by the SARS-CoV-2 virus.

Katalin "Kati" Karikó is a Hungarian-American biochemist who specializes in ribonucleic acid (RNA)-mediated mechanisms, particularly in vitro-transcribed messenger RNA (mRNA) for protein replacement therapy. Karikó laid the scientific groundwork for mRNA vaccines, overcoming major obstacles and skepticism in the scientific community. Karikó received the Nobel Prize in Physiology or Medicine in 2023 for her work, along with American immunologist Drew Weissman.
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company BioNTech. For its development, BioNTech collaborated with the American company Pfizer to carry out clinical trials, logistics, and manufacturing. It is authorized for use in humans to provide protection against COVID-19, caused by infection with the SARS-CoV-2 virus. The vaccine is given by intramuscular injection. It is composed of nucleoside-modified mRNA (modRNA) that encodes a mutated form of the full-length spike protein of SARS-CoV-2, which is encapsulated in lipid nanoparticles. Initial guidance recommended a two-dose regimen, given 21 days apart; this interval was subsequently extended to up to 42 days in the United States, and up to four months in Canada.
A nucleoside-modified messenger RNA (modRNA) is a synthetic messenger RNA (mRNA) in which some nucleosides are replaced by other naturally modified nucleosides or by synthetic nucleoside analogues. modRNA is used to induce the production of a desired protein in certain cells. An important application is the development of mRNA vaccines, of which the first authorized were COVID-19 vaccines.

ALC-0315 is a synthetic lipid. A colorless oily material, it has attracted attention as a component of the SARS-CoV-2 vaccine, BNT162b2, from BioNTech and Pfizer. Specifically, it is one of four components that form lipid nanoparticles (LNPs), which encapsulate and protect the otherwise fragile mRNA that is the active ingredient in these drugs. These nanoparticles promote the uptake of therapeutically effective nucleic acids such as oligonucleotides or mRNA both in vitro and in vivo.
Distearoylphosphatidylcholine is a phosphatidylcholine, a kind of phospholipid. It is a natural constituent of cell membranes, eg. soybean phosphatidylcholines are mostly different 18-carbon phosphatidylcholines, and their hydrogenation results in 85% DSPC. It can be used to prepare lipid nanoparticles which are used in mRNA vaccines, In particular, it forms part of the drug delivery system for the Moderna and Pfizer COVID-19 vaccines.
ALC-0159 is a PEG/lipid conjugate, specifically, it is the N,N-dimyristylamide of 2-hydroxyacetic acid, O-pegylated to a PEG chain mass of about 2 kilodaltons. It is a non-ionic surfactant by its nature. It has been deployed in the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine that contains the active ingredient tozinameran.

Drew Weissman is an American physician and immunologist known for his contributions to RNA biology. Weissman is the inaugural Roberts Family Professor in Vaccine Research, director of the Penn Institute for RNA Innovation, and professor of medicine at the Perelman School of Medicine at the University of Pennsylvania (Penn).
SM-102 is a synthetic amino lipid which is used in combination with other lipids to form lipid nanoparticles. These are used for the delivery of mRNA-based vaccines, and in particular SM-102 forms part of the drug delivery system for the Moderna COVID-19 vaccine.

AWcorna, originally termed ARCoV and also known as the Walvax COVID-19 vaccine, is an mRNA COVID-19 vaccine developed by Walvax Biotechnology, Suzhou Abogen Biosciences, and the PLA Academy of Military Science. In contrast to other mRNA COVID-19 vaccines, such as those by Pfizer-BioNTech and Moderna, this vaccine primarily targets the SARS-CoV-2 receptor-binding domain of the spike protein, rather than the entire spike protein. It is approved for Phase III trials in China, Mexico, Indonesia, and Nepal.

mRNA-1283 is a COVID-19 vaccine candidate developed by Moderna.
Pieter Rutter Cullis is a Canadian physicist and biochemist known for his contributions to the field of lipid nanoparticles (LNP). Cullis and co-workers have been responsible for fundamental advances in the development of nanomedicines employing lipid nanoparticle (LNP) technology for cancer therapies, gene therapies and vaccines. This work has contributed to five drugs that have received clinical approval by the US Food and Drug Agency (FDA), the European Medicines Agency, and Health Canada.
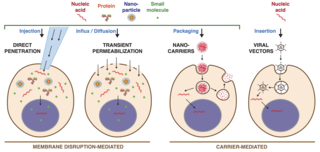
Intracellular delivery is the process of introducing external materials into living cells. Materials that are delivered into cells include nucleic acids, proteins, peptides, impermeable small molecules, synthetic nanomaterials, organelles, and micron-scale tracers, devices and objects. Such molecules and materials can be used to investigate cellular behavior, engineer cell operations or correct a pathological function.

Acuitas Therapeutics Inc. is a Canadian biotechnology company based in Vancouver, British Columbia. The company was established in February 2009 to specialize in the development of delivery systems for nucleic acid therapeutics based on lipid nanoparticle (LNP) technology, a key component of the mRNA vaccines deployed for COVID-19.














